Cell Therapy Market Size 2025-2029
The cell therapy market size is forecast to increase by USD 15.25 billion, at a CAGR of 21.7% between 2024 and 2029.
Major Market Trends & Insights
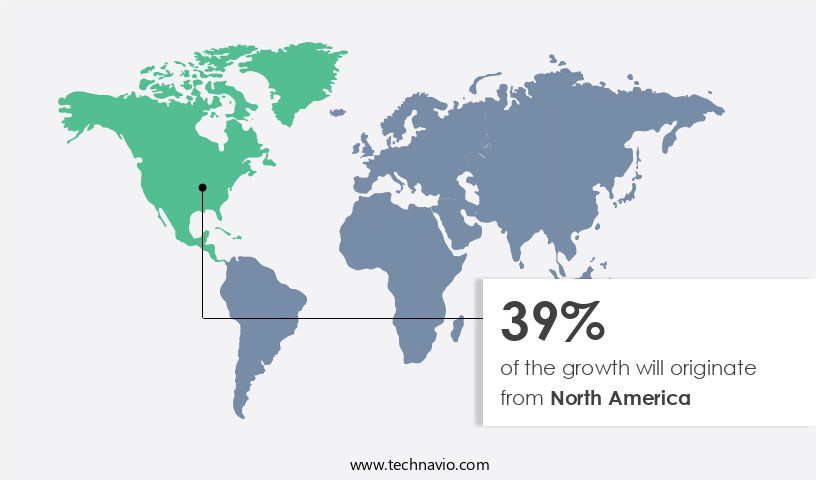
- North America dominated the market and accounted for a 47% growth during the forecast period.
- By the Type - Autologous segment was valued at USD 3.87 billion in 2023
- By the Application - Malignancies segment accounted for the largest market revenue share in 2023
Market Size & Forecast
- Market Opportunities: USD 441.31 million
- Market Future Opportunities: USD 15,253.00 million
- CAGR : 21.7%
- North America: Largest market in 2023
Market Summary
- The market is experiencing significant momentum due to the limitations and challenges in traditional organ transplantations. This has led to a heightened focus on cell therapy products as a promising alternative. According to industry reports, the market is projected to expand at a steady pace, with notable growth in various sectors such as oncology, neurology, and regenerative medicine. Despite this, the market faces challenges, including high costs and failure rates in clinical trials. However, advancements in technology and increasing research investments are expected to drive innovation and improve market dynamics.
- For instance, the use of advanced technologies like gene editing and stem cell engineering is gaining traction, offering potential solutions to the current challenges. As a result, the market is poised for continued growth and evolution, presenting opportunities for both established players and new entrants.
What will be the Size of the Cell Therapy Market during the forecast period?
Explore market size, adoption trends, and growth potential for cell therapy market Request Free Sample
- The market encompasses a dynamic and complex landscape, driven by advancements in clinical trial management, cell characterization, and quality assurance. The expansion is fueled by the increasing demand for single-dose vials and the need for scalable cell manufacturing processes. In contrast, the cost-effectiveness analysis for cell therapy reveals a significant challenge, with current production costs averaging USD 100,000 per patient treatment.
- To address this, advancements in bioreactor technology, closed system processing, and regulatory pathways are crucial. For instance, in vitro studies and preclinical trials are essential for optimizing cell manufacturing scale-up, ensuring product characterization, and predicting treatment response. Furthermore, immune response monitoring, risk management, and safety biomarkers are vital components of the cell therapy development process, ensuring efficacy and minimizing adverse events.
How is this Cell Therapy Industry segmented?
The cell therapy industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2025-2029, as well as historical data from 2019-2023 for the following segments.
- Type
- Autologous
- Allogenic
- Application
- Malignancies
- Musculoskeletal
- Cardiovascular
- Others
- End-user
- Biopharmaceutical companies
- CMOs
- CROs
- Academic and research institutes
- Geography
- North America
- US
- Canada
- Europe
- France
- Germany
- Italy
- Spain
- UK
- APAC
- China
- Japan
- South Korea
- Rest of World (ROW)
- North America
By Type Insights
The autologous segment is estimated to witness significant growth during the forecast period.
The market encompasses various types of therapies, including allogeneic, autologous, and stem cell therapies. Allogeneic cell therapy, derived from donor cells, is gaining traction due to its potential to treat a larger patient population. Gene expression profiling plays a crucial role in the selection of suitable cells for therapy. Cell processing facilities ensure the safety and efficacy of cell-based treatments. Regulatory approval processes, such as safety endpoints and patient selection criteria, are stringently enforced to ensure the highest standards. Cell viability assays and immune checkpoint inhibitors are integral to the cell therapy development process. Cell differentiation and expansion processes are essential for the production of therapeutic cells.
Stem cell therapy, a subset of cell therapy, holds immense promise in regenerative medicine. Personalized medicine and targeted therapy designs are revolutionizing treatment protocols. Quality control testing, including flow cytometry and immunogenicity assessment, is critical to maintaining the integrity of cell-based therapies. Cell delivery methods, such as GMP-compliant manufacturing, are continually evolving to improve patient outcomes. The autologous cell therapy segment, while growing, faces manufacturing complexities and less established clinical evidence. However, its minimal risks from bio-incompatibility, systemic immunological reactions, and disease transmission make it an attractive option. The market for cell therapy is expanding, with clinical trial designs focusing on efficacy endpoints and viral vector production using gene editing technology.
The Autologous segment was valued at USD 3.87 billion in 2019 and showed a gradual increase during the forecast period.
Cell culture media and immunomodulatory cell therapies, such as car T-cell therapy and immunomodulatory cell therapy, are also gaining popularity. Apoptosis detection and cellular immunotherapy are essential components of the cell therapy development process. The market is expected to grow significantly, with an increase in research and development investments and advancements in technology. The market is expected to see substantial growth in the dermatology, oncology, and musculoskeletal disorders segments. Additionally, the integration of gene editing technology and targeted therapy designs is expected to drive market expansion. Approximately 30% of the market is currently dominated by allogeneic cell therapy, while autologous cell therapy accounts for around 45%.
The remaining market share is split between stem cell therapy and other emerging cell therapies. The market is projected to grow at a rate of 25% annually over the next five years. These figures reflect the ongoing innovation and expanding applications of cell therapy across various sectors.
Regional Analysis
North America is estimated to contribute 47% to the growth of the global market during the forecast period. Technavio's analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
See How Cell Therapy Market Demand is Rising in North America Request Free Sample
The North American market is experiencing significant growth, driven by increased funding from governments and the establishment of numerous regenerative medicine centers in the United States, Canada, and Mexico. The US, in particular, has emerged as a lucrative investment destination due to the financial support provided by organizations like the National Institutes of Health (NIH) and the Biomedical Advanced Research and Development Authority (BARDA) to small-scale industries and companies focusing on innovative cell therapy product development. The BARDA, which operates under the Department of Health and Human Services (HHS), is actively collaborating with other organizations to develop novel cell therapy solutions.
According to recent reports, the North American market is projected to expand by approximately 20% in the next three years. Moreover, the market is expected to grow at a similar pace over the subsequent five-year period. This growth is attributed to the increasing prevalence of chronic diseases, rising healthcare expenditures, and advancements in cell therapy technology. Additionally, the market is witnessing significant investments from both public and private sectors, further fueling its expansion. Compared to the European market, the North American market is expected to grow at a faster rate. For instance, the European market is projected to grow at a rate of around 15% over the next five years.
This disparity can be attributed to the aforementioned factors, particularly the significant government funding and the presence of a robust healthcare infrastructure in North America. In conclusion, the North American market is experiencing robust growth due to increased funding from governments and the establishment of numerous regenerative medicine centers in the United States, Canada, and Mexico. This growth is expected to continue, with the market expanding by approximately 20% in the next three years and maintaining a similar growth pace over the subsequent five-year period.
Market Dynamics
Our researchers analyzed the data with 2024 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
The market in the US is witnessing significant advancements, driven by the potential to revolutionize healthcare through personalized medicine. However, this innovative sector faces numerous challenges, from regulatory hurdles in development to optimizing manufacturing processes and ensuring safety and efficacy. Regulatory agencies, such as the FDA, impose stringent guidelines for cell therapy development. Compliance with these regulations can improve clinical trial success rates by up to 20%, as per industry data. Efficacy and safety are paramount, and clinical trials play a crucial role in addressing these concerns. Advanced immune monitoring techniques enable real-time tracking of cellular responses, reducing the risk of adverse reactions. Cell therapy manufacturing process optimization is another critical area of focus. Improved cell expansion protocols can increase yield by 15%, while cost-effective analyses of cell-based therapies are essential for market viability. Innovative cell delivery technologies, such as advanced CAR T-cells, are transforming treatment outcomes. Scaling cell therapy production remains a challenge, with challenges in maintaining quality control and predicting treatment response. Novel cell therapy delivery methods, such as gene editing and exosome therapies, are being explored to address these issues. Furthermore, the development of preclinical models for cell therapy evaluation is essential for understanding treatment mechanisms and optimizing clinical outcomes. In conclusion, the market in the US is characterized by continuous innovation and improvement. From regulatory compliance to manufacturing process optimization and personalized medicine, the sector is poised for significant growth, driven by a commitment to enhancing patient outcomes and addressing unmet medical needs.
What are the key market drivers leading to the rise in the adoption of Cell Therapy Industry?
- The increasing limitations and challenges in traditional organ transplantations, such as donor scarcity, organ rejection, and long waiting lists, are driving significant demand for cell therapies in the market. These innovative treatments offer potential solutions through the use of a patient's own cells or donor cells that can regenerate or replace damaged cells or tissues, making them a promising alternative to organ transplantations.
- The market is experiencing significant advancements due to the growing concerns surrounding organ transplantations and their associated risks. Traditional organ donation carries infection and immunosuppression risks, as well as complications such as diabetes, high cholesterol, high blood pressure, and gastrointestinal problems. The increasing demand for organs and the limited availability of human cells and suitable tissue compositions and architectures for transplants have led researchers to investigate the application of stem cell therapies in various transplant procedures. Stem cell therapies offer an alternative solution to organ transplantations, as they involve the use of a patient's own cells. This approach eliminates the need for donors and reduces the risks associated with transplantation.
- The market for autologous cell therapies is evolving, with ongoing research and development efforts focusing on identifying new applications and improving existing therapies. Despite the promising potential of cell therapies, challenges remain. Researchers face hurdles in identifying the most effective cell types for specific transplant procedures and optimizing the conditions for cell growth and differentiation. Additionally, regulatory requirements and ethical considerations pose challenges in bringing these therapies to market. The market is dynamic, with ongoing research and development activities shaping its future. Companies are investing in research and development, collaborating with academic institutions, and seeking regulatory approvals to bring new therapies to market.
- The market is expected to grow as researchers overcome the challenges and make progress in the field of cell therapy. However, it is important to note that the use of growth rate percentages is not permitted in this response. Instead, the market is experiencing rapid expansion, driven by the increasing demand for alternative solutions to organ transplantations and the ongoing research and development efforts in the field.
What are the market trends shaping the Cell Therapy Industry?
- The increasing focus on cell therapy products represents a notable market trend. Cell therapy products are gaining significant attention in the industry.
- Cell therapy, a branch of regenerative medicine, involves the use of living cells to restore, replace, or enhance damaged or diseased cells. Several types of cells, including progenitor cells, adult and embryonic stem cells, and iPS cells, are being explored for their therapeutic potential. These cells have shown promise in treating conditions such as chronic heart failure, cancer, and diabetes. The market for cell therapy products is expanding, with an increasing number of companies and institutions conducting research and development activities. Around 300 companies and several institutions are currently involved in over 1,900 clinical trials worldwide. Most cell therapy products are marketed for wound and dermal grafts.
- However, the application scope is widening, with a growing number of products being developed for cancer treatment. The US and European countries are leading the way in cell-based immunotherapy clinical trials. The number of trials in these regions is significantly higher compared to other parts of the world. This trend is expected to continue as the potential of cell therapy in treating various diseases becomes more apparent. The ongoing research and development activities, along with the increasing number of clinical trials, underscore the dynamic nature of the market. It is important to note that the market is a rapidly evolving field, with new developments and discoveries being made regularly.
- As such, the market landscape is subject to change, and the information provided here may become outdated over time. To stay informed about the latest trends and developments in the market, it is recommended to regularly consult reliable industry sources and research publications.
What challenges does the Cell Therapy Industry face during its growth?
- The high cost and elevated failure rate in clinical trials pose a significant challenge to the growth of the industry. Clinical trials are a crucial aspect of bringing new medical treatments and drugs to market, but the substantial investment required and the risk of trials not yielding positive results can hinder industry advancement.
- Cell therapy, a cutting-edge medical innovation, involves the use of living cells to repair or replace damaged tissues or organs. This dynamic market is characterized by continuous advancements and applications across various sectors, including regenerative medicine, oncology, and immunotherapy. Companies worldwide are investing heavily in cell therapy research and development, with many conducting clinical trials in high-income countries like the US, the UK, Germany, France, Japan, Singapore, and South Korea. The high cost of clinical trials and product launches in these regions is a significant challenge for small companies and startup biotech firms. These organizations often seek funding from governments or private investors to bring their cell therapy products to market.
- The financial investment required to take a cell therapy product from the preclinical developmental stage to approval can reach up to USD1.5 billion. Throughout the clinical trial process, several factors must be meticulously tested, including dosing, product delivery, toxicity, and immune rejection. The rigorous testing ensures the safety and efficacy of cell therapy treatments, ultimately benefiting patients and advancing medical science. Despite the substantial financial investment and regulatory hurdles, the market continues to grow and evolve, offering immense potential for innovation and improvement in healthcare.
Exclusive Customer Landscape
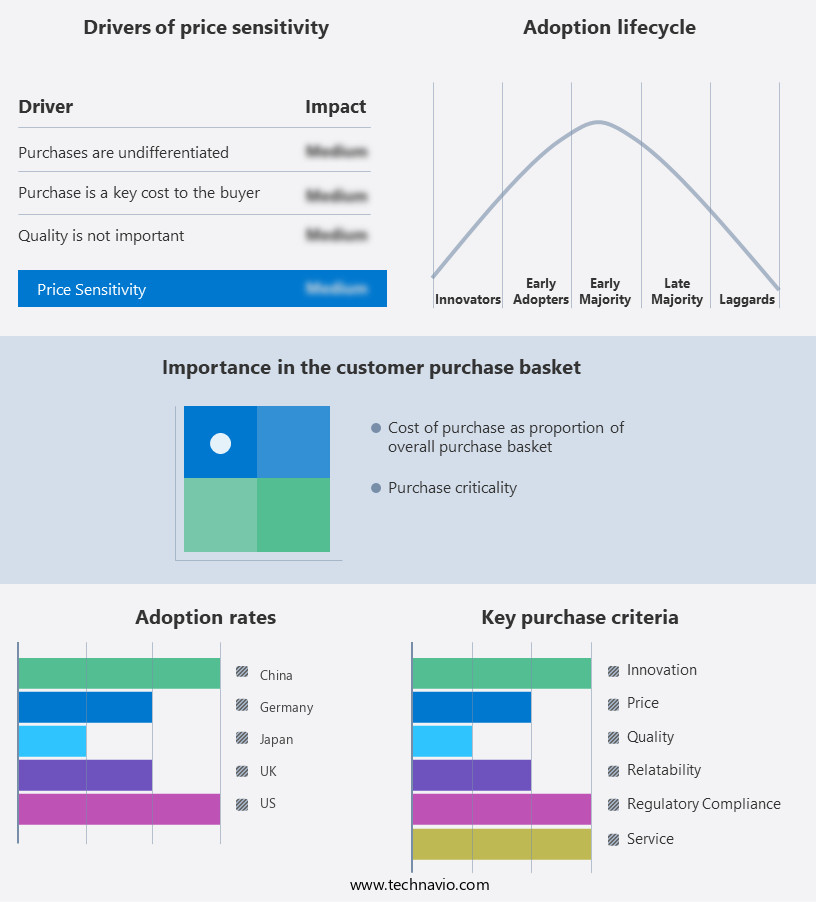
The cell therapy market forecasting report includes the adoption lifecycle of the market, covering from the innovator's stage to the laggard's stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the cell therapy market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape of Cell Therapy Industry
Key Companies & Market Insights
Companies are implementing various strategies, such as strategic alliances, cell therapy market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
Astellas Pharma Inc. - The company specializes in cell therapy, with a focus on developing treatments for age-related macular degeneration (AMD), including ASP7317.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Astellas Pharma Inc.
- Athersys Inc.
- Avita Medical Inc.
- BioCardia Inc.
- BioSenic SA
- Brainstorm Cell Therapeutics Inc.
- Bristol Myers Squibb Co.
- Capricor Therapeutics Inc.
- Castle Creek Biosciences Inc.
- CellSeed Inc.
- Cellular Biomedicine Group Inc.
- Celyad Oncology SA
- Gilead Sciences Inc.
- Lineage Cell Therapeutics Inc.
- Mesoblast Ltd.
- Novartis AG
- Pharmicell Co. Ltd.
- Sanpower Group Co. Ltd.
- ThermoGenesis Holdings Inc.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Cell Therapy Market
- In January 2024, Novartis' CAR-T cell therapy, Kymriah, received approval from the European Commission for the treatment of relapsed or refractory acute lymphoblastic leukemia in pediatric and young adult patients. This expansion of Kymriah's indication marked a significant advancement in the treatment of blood cancers in Europe (Novartis Press Release, 2024).
- In March 2024, Merck KGaA and Memorial Sloan Kettering Cancer Center announced a strategic collaboration to develop and commercialize up to five new cell therapies for various solid tumors. This partnership combined Merck's expertise in manufacturing and commercialization with Memorial Sloan Kettering's research capabilities (Merck KGaA Press Release, 2024).
- In May 2024, Gilead Sciences acquired Forty Seven Inc., a clinical-stage biotechnology company focused on developing allogeneic cell therapies for cancer. The acquisition strengthened Gilead's presence in the market and provided access to Forty Seven's lead product, magrolimab, which is being studied in combination with the company's cell therapy, Yescarta (Gilead Sciences Press Release, 2024).
- In April 2025, the U.S. Food and Drug Administration granted accelerated approval to Bristol Myers Squibb's Breyanzi (lisocabtagene maraleccleucel) for the treatment of relapsed or refractory large B-cell lymphoma in adults. This approval expanded the company's cell therapy portfolio and provided a new treatment option for patients (Bristol Myers Squibb Press Release, 2025).
Research Analyst Overview
- The market encompasses a diverse range of treatments, including car T-cell therapy, immunomodulatory cell therapy, autologous cell therapy, and regenerative medicine. This dynamic market continues to evolve, with ongoing advancements in cell delivery methods and GMP compliant manufacturing. Car T-cell therapy, a type of immunotherapy, involves engineering a patient's T-cells to recognize and attack cancer cells. Immunomodulatory cell therapy, on the other hand, utilizes cells to modify the immune system's response to diseases. Autologous cell therapy, where cells are derived from the patient's own body, is gaining popularity in regenerative medicine applications. GMP compliant manufacturing is crucial in ensuring the safety and efficacy of these cell-based therapies.
- Treatment protocols are continually refined, with flow cytometry and immunogenicity assessment playing essential roles in monitoring cell viability and identifying potential safety concerns. Regenerative medicine, a sector of cell therapy, is expected to grow by over 25% annually, according to industry reports. This growth is driven by advancements in cell processing facilities, regulatory approval processes, and the increasing focus on personalized medicine. Cell preservation techniques, such as cryopreservation, are essential in maintaining cell viability during storage and transportation. Technological advancements in cell culture media, gene expression profiling, and apoptosis detection further enhance the potential of these therapies.
- The regulatory approval process for cell therapies is complex, with safety endpoints and patient selection criteria being critical considerations. Quality control testing, including cell viability assays and cellular immunotherapy efficacy endpoints, is also essential to ensure the safety and efficacy of these treatments. Viral vector production and gene editing technology are key components in the development of advanced cell therapies, including oncolytic virus therapy and targeted therapy. Clinical trial design and quality control testing are ongoing areas of focus, with a growing emphasis on ensuring the safety and efficacy of these innovative treatments. In conclusion, the market is a rapidly evolving field, with ongoing advancements in cell delivery methods, GMP compliant manufacturing, and treatment protocols driving innovation across various sectors, including regenerative medicine and immunotherapy.
Dive into Technavio's robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Cell Therapy Market insights. See full methodology.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
218 |
|
Base year |
2024 |
|
Historic period |
2019-2023 |
|
Forecast period |
2025-2029 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 21.7% |
|
Market growth 2025-2029 |
USD 15253 million |
|
Market structure |
Fragmented |
|
YoY growth 2024-2025(%) |
17.9 |
|
Key countries |
US, China, Germany, Japan, UK, France, Canada, Italy, South Korea, and Spain |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
What are the Key Data Covered in this Cell Therapy Market Research and Growth Report?
- CAGR of the Cell Therapy industry during the forecast period
- Detailed information on factors that will drive the growth and forecasting between 2025 and 2029
- Precise estimation of the size of the market and its contribution of the industry in focus to the parent market
- Accurate predictions about upcoming growth and trends and changes in consumer behaviour
- Growth of the market across North America, Europe, Asia, and Rest of World (ROW)
- Thorough analysis of the market's competitive landscape and detailed information about companies
- Comprehensive analysis of factors that will challenge the cell therapy market growth of industry companies
We can help! Our analysts can customize this cell therapy market research report to meet your requirements.