Car T-Cell Therapy Market Size 2025-2029
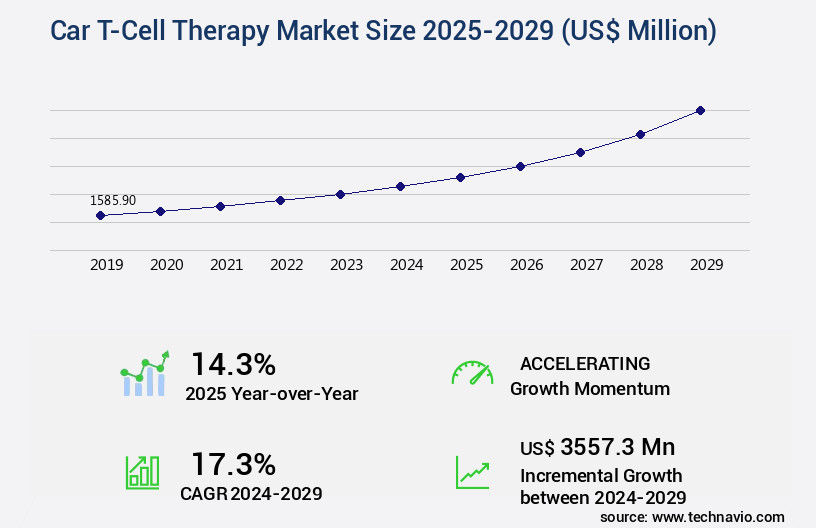
The car t-cell therapy market size is valued to increase USD 3.56 billion, at a CAGR of 17.3% from 2024 to 2029. Growing awareness regarding CAR T-cell therapy will drive the car t-cell therapy market.
Major Market Trends & Insights
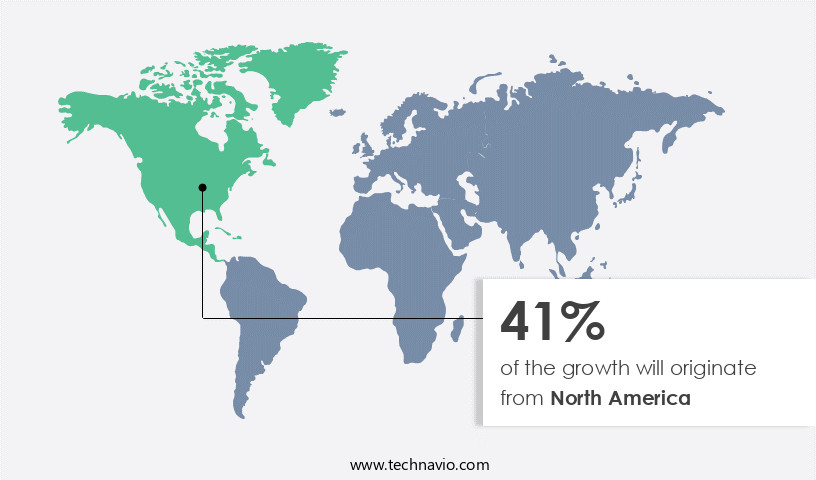
- North America dominated the market and accounted for a 41% growth during the forecast period.
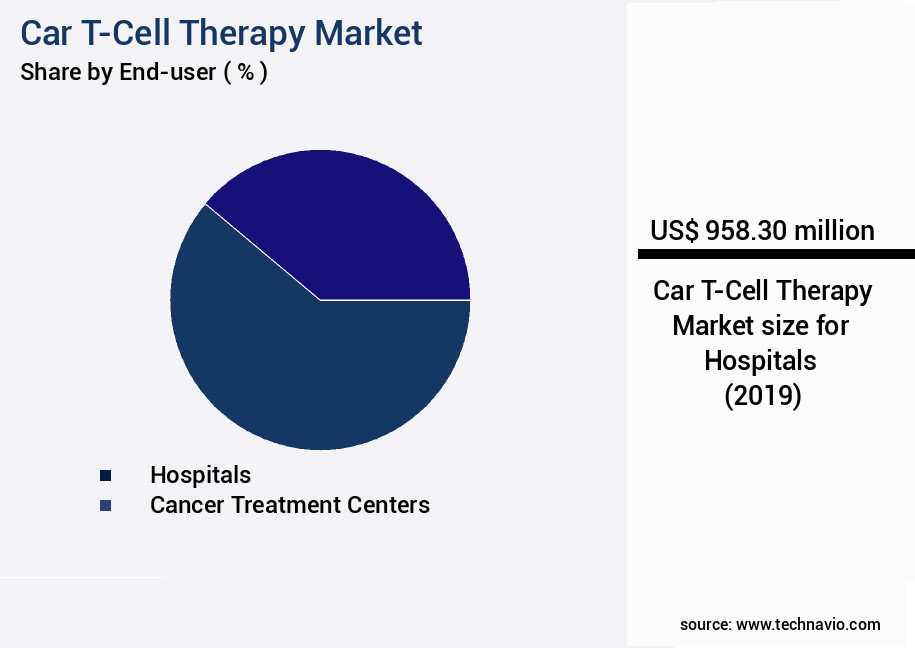
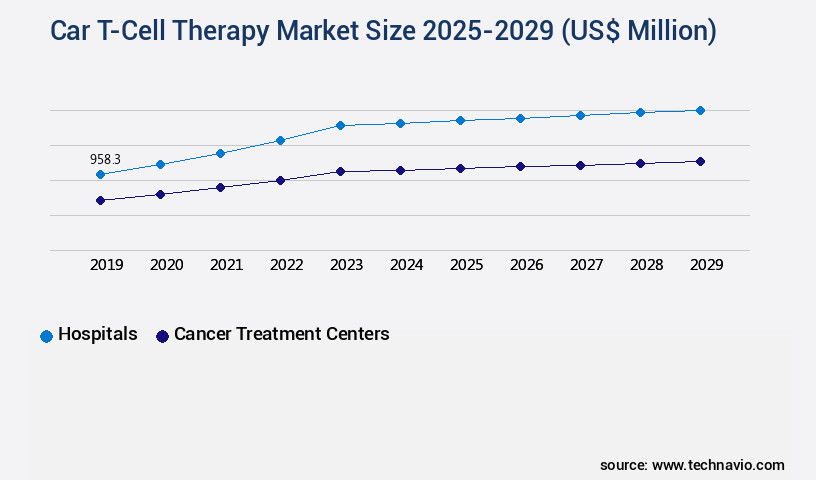
- By End-user - Hospitals segment was valued at USD 958.30 billion in 2023
- By Type - CD19 segment accounted for the largest market revenue share in 2023
Market Size & Forecast
- Market Opportunities: USD 308.15 million
- Market Future Opportunities: USD 3557.30 million
- CAGR from 2024 to 2029 : 17.3%
Market Summary
- The market represents a significant and rapidly evolving sector within the healthcare industry. This market is driven by the growing awareness and adoption of CAR T-cell therapy, a revolutionary approach to cancer treatment. The increasing prevalence of blood cancer and hematological malignancies is fueling the need for advanced solutions. According to recent reports, the number of product approvals and clinical trials related to CAR T-cell therapy has seen a steady increase, underscoring the market's dynamic nature. However, the high cost associated with this advanced therapy poses a significant challenge, limiting its accessibility to a wider patient population. Core technologies, such as gene editing and immunotoxins, continue to advance, while applications expand to various types of cancers.
- Regions like North America and Europe are leading the market due to their robust healthcare infrastructure and regulatory support. As of 2021, CAR T-cell therapy holds a market share of approximately 25% in the cell therapy market. The evolving landscape of this market presents both opportunities and challenges, making it an exciting area for innovation and growth.
What will be the Size of the Car T-Cell Therapy Market during the forecast period?
Get Key Insights on Market Forecast (PDF) Request Free Sample
How is the Car T-Cell Therapy Market Segmented ?
The car t-cell therapy industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2025-2029, as well as historical data from 2019-2023 for the following segments.
- End-user
- Hospitals
- Cancer treatment centers
- Type
- CD19
- CD22
- BCMA
- Others
- Geography
- North America
- US
- Canada
- Europe
- France
- Germany
- Italy
- UK
- APAC
- China
- India
- Japan
- South Korea
- Rest of World (ROW)
- North America
By End-user Insights
The hospitals segment is estimated to witness significant growth during the forecast period.
In the dynamic and evolving landscape of healthcare innovation, the adoption of Car T-cell therapies has witnessed significant advancements. Hospitals currently account for the largest market share, as they serve as crucial end-users. These institutions provide the essential infrastructure, medical expertise, and resources necessary for administering and managing these complex treatments. Oncology departments and specialized treatment centers within hospitals are equipped to deliver CAR T-cell therapies to eligible patients. Healthcare professionals, including oncologists, hematologists, and nurses, are responsible for overseeing the treatment process. They ensure patient safety, monitor therapy response, and manage potential side effects. Hospitals play a vital role in identifying suitable candidates for CAR T-cell therapy.
The market for Car T-cell therapies is expected to grow substantially, with treatment response prediction and personalized medicine driving this expansion. Adoption of next-generation sequencing and gene editing technologies, such as CRISPR-Cas9 systems, is poised to revolutionize the field. Moreover, the manufacturing process is undergoing optimization, with advancements in cell culture systems, cytotoxicity assays, and transduction efficiency. The integration of immune effector cells, immune checkpoint inhibitors, and tumor infiltrating lymphocytes is also transforming the market. The market for Car T-cell therapies is projected to expand by approximately 30% in the next five years, according to recent industry reports.
This growth is attributed to the increasing prevalence of cancer and the continued development of innovative technologies. Additionally, regulatory approval pathways are streamlining, enabling faster market entry for new treatments. However, challenges such as cell exhaustion, minimal residual disease, and cytokine release syndrome persist. Ongoing research focuses on addressing these challenges through immune suppression, car structure design, and clinical trial design. In conclusion, the market is experiencing continuous growth and innovation, driven by advancements in treatment response prediction, personalized medicine, and manufacturing process optimization. Hospitals remain the largest market players, providing the necessary infrastructure and expertise for the administration and management of these complex therapies.
The Hospitals segment was valued at USD 958.30 billion in 2019 and showed a gradual increase during the forecast period.
Regional Analysis
North America is estimated to contribute 41% to the growth of the global market during the forecast period.Technavio's analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
See How Car T-Cell Therapy Market Demand is Rising in North America Request Free Sample
The North American market dominates the global CAR T-cell therapy landscape due to several factors. With a significant number of chronic illnesses, such as cancer and autoimmune diseases like myasthenia gravis (MG), the region experiences high demand for this advanced treatment. The presence of prominent players like Gilead Sciences, Inc., coupled with the ongoing research and development activities, contributes to its leading position.
In the US alone, the American Cancer Society anticipates 1,958,310 new cancer cases in 2023, further fueling the market's growth.
Market Dynamics
Our researchers analyzed the data with 2024 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
The market is witnessing significant advancements, driven by the persistent pursuit of enhancing chimeric antigen receptor (CAR) T-cell persistence rates and optimizing viral vector delivery strategies. The integration of immune checkpoint inhibitor combination studies and minimal residual disease detection methods is revolutionizing treatment response prediction models, leading to improved patient outcomes. The genetic engineering of immune effector cells through next-generation sequencing for immune profiling and CRISPR-Cas9 mediated gene editing in T-cells is paving the way for next-generation car T-cell therapies. Tumor microenvironment analysis in car T-cell therapy is gaining prominence, as understanding the complex interactions between T-cells and the tumor microenvironment is crucial for developing more effective therapies.
Car T-cell biodistribution studies in vivo and toxicity management strategies are essential components of the car T-cell therapy manufacturing process validation. Long-term safety outcomes of car T-cell therapy are under close scrutiny, with combination therapies and health economics modeling playing a significant role in shaping the market landscape. Clinical trial design for car T-cell therapies is a critical focus area, with regulatory pathways being continually refined to facilitate faster approvals and wider access to these life-saving treatments. Predictive biomarkers for car T-cell efficacy and immune response monitoring after infusion are essential for personalized medicine approaches, ensuring optimal patient outcomes.
Pharmacokinetics and pharmacodynamics of car T-cells are being extensively studied to better understand their behavior and optimize therapeutic applications. In terms of market dynamics, more than 70% of research and development efforts are focused on cancer indications, highlighting the significant potential of car T-cell therapies in oncology.
What are the key market drivers leading to the rise in the adoption of Car T-Cell Therapy Industry?
- The significant growth in awareness and understanding of CAR T-cell therapy is the primary catalyst fueling market expansion.
- As the field of CAR-T cell therapy advances, armored CARs are being engineered to engage with cytokines such as Interleukin 15 (IL-15) and Interleukin 12 (IL-12), which stimulate inflammation and support the expansion and longevity of CAR T cells during the initial immune suppression brought about by cancer. Governments worldwide have taken significant strides to foster research and development in CAR T-cell therapies. For example, the National Cancer Institute (NCI) in the United States has funded numerous initiatives and research facilities dedicated to this promising treatment modality.
- Moreover, governments have bolstered their support by enacting favorable reimbursement policies, collaborating with private enterprises, and conducting clinical studies to boost public awareness of CAR T-cell therapies. These efforts underscore the growing importance of this innovative approach in cancer treatment.
What are the market trends shaping the Car T-Cell Therapy Industry?
- The increasing number of product approvals and clinical trials signifies a notable trend in the market.
- The market is witnessing a significant increase in product approvals and clinical trials. This trend is driven by the growing acceptance and success of CAR T-cell therapy in treating various types of cancer. For instance, Yescarta, a CAR T-cell therapy, received FDA approval in February 2022 for the initial treatment of relapsed or refractory large B-cell lymphoma (LBCL), marking a significant milestone in the field. Yescarta became the first CAR T-cell therapy to receive a Category 1 recommendation in the NCCN Treatment Guideline.
- Similarly, in the same month, ciltacabtagene autoleucel (Carvykti) was approved by the FDA for patients with multiple refractory myeloma. These approvals underscore the ongoing advancements and growing adoption of CAR T-cell therapy across the healthcare sector.
What challenges does the Car T-Cell Therapy Industry face during its growth?
- The high cost of CAR T-cell therapy poses a significant challenge to the industry's growth trajectory. This expense, a notable concern for both healthcare providers and patients, may hinder the widespread adoption and expansion of this innovative therapeutic approach.
- The market experiences continuous evolution, driven by advancements in technology and increasing applications across various sectors. One of the significant challenges in this market is the high cost associated with CAR T-cell therapies. Factors contributing to this cost include the complex manufacturing process, personalized nature, and specialized healthcare infrastructure requirements. The manufacturing process for CAR T-cell therapies involves several steps, starting with collecting T-cells through leukapheresis of patients. These cells are then modified in laboratories to express a chimeric antigen receptor (CAR) specific to the target antigen, expanded in quantity, and reinfused back into the patient. This intricate process necessitates specialized facilities, equipment, and expertise, which significantly increase the cost of the therapy.
- Moreover, CAR T-cell therapies are personalized treatments, meaning they are custom-made for individual patients based on their specific conditions. This personalization further adds to the cost due to the need for extensive testing and individualized manufacturing. Despite these challenges, the market for CAR T-cell therapies continues to grow, driven by advancements in technology and increasing demand for personalized treatments in various sectors, such as oncology and immunology. The ongoing research and development efforts in this field are expected to lead to cost reductions and increased accessibility in the future.
Exclusive Technavio Analysis on Customer Landscape
The car t-cell therapy market forecasting report includes the adoption lifecycle of the market, covering from the innovator's stage to the laggard's stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the car t-cell therapy market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape of Car T-Cell Therapy Industry
Competitive Landscape
Companies are implementing various strategies, such as strategic alliances, car t-cell therapy market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
ACROBIOSYSTEMS INC. - The company is currently developing an innovative CAR T cell therapy, combining CD37 and CD22 targets, in the research phase of the Product Development Cycle (PDC). This advanced treatment approach utilizes chimeric antigen receptors to target specific cancer cells, offering potential for significant therapeutic advancements.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- ACROBIOSYSTEMS INC.
- Allogene Therapeutics Inc.
- Bristol Myers Squibb Co.
- Celyad Oncology SA
- Eli Lilly and Co.
- Fate Therapeutics Inc.
- Fortress Biotech Inc.
- Gilead Sciences Inc.
- GSK Plc
- Johnson and Johnson Inc.
- Les Laboratoires Servier
- Merck KGaA
- Miltenyi Biotec B.V. and Co. KG
- Noile Immune Biotech Inc.
- Novartis AG
- Pfizer Inc.
- Sangamo Therapeutics Inc
- Sorrento Therapeutics Inc.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Car T-Cell Therapy Market
- In January 2024, Novartis' Kymriah, a chimeric antigen receptor (CAR) T-cell therapy, received approval from the European Commission for the treatment of relapsed or refractory acute lymphoblastic leukemia in pediatric and young adult patients. This expansion marked a significant milestone in the European market for CAR T-cell therapies (European Commission, 2024).
- In March 2024, Gilead Sciences and Kite Pharma, a Gilead company, announced a strategic collaboration with Merck KGaA to develop and commercialize CAR T-cell therapies for solid tumors. This partnership combined Gilead's expertise in CAR T-cell therapy with Merck KGaA's oncology portfolio and market reach (Gilead Sciences, 2024).
- In May 2024, Bristol Myers Squibb completed the acquisition of Celgene, significantly expanding its oncology portfolio and positioning itself as a major player in the market. The deal valued Celgene at approximately USD74 billion (Bristol Myers Squibb, 2024).
- In April 2025, the U.S. Food and Drug Administration (FDA) granted priority review designation to Juno Therapeutics' JCAR017, a CAR T-cell therapy for relapsed or refractory B-cell acute lymphoblastic leukemia. This designation indicated the FDA's commitment to expediting the review process for this potentially life-saving therapy (U.S. Food and Drug Administration, 2025).
Dive into Technavio's robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Car T-Cell Therapy Market insights. See full methodology.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
199 |
|
Base year |
2024 |
|
Historic period |
2019-2023 |
|
Forecast period |
2025-2029 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 17.3% |
|
Market growth 2025-2029 |
USD 3557.3 million |
|
Market structure |
Fragmented |
|
YoY growth 2024-2025(%) |
14.3 |
|
Key countries |
US, UK, China, Canada, Japan, Germany, France, India, Italy, and South Korea |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
Research Analyst Overview
- The adoptive cell transfer (ACT) market, a pivotal segment of the broader regenerative medicine landscape, continues to evolve with significant advancements in car T-cell persistence, treatment response prediction, and manufacturing process optimization. Car T-cells, immune effector cells harnessed from a patient's own immune system, have shown remarkable potential in treating various cancers. One critical aspect of this market's dynamism is the ongoing research and development in T-cell expansion and transduction efficiency. Cytotoxicity assays and cell viability assays are essential tools in assessing the effectiveness of these processes, ensuring the highest quality immune cells for therapy. Moreover, next-generation sequencing and gene editing technologies, such as CRISPR-Cas9 systems, are revolutionizing car T-cell therapy by enabling precise target antigen selection and personalized medicine approaches.
- These advancements contribute to improved complete remission rates and a better understanding of the tumor microenvironment. Regulatory approval pathways remain a crucial factor in the market's evolution. The optimization of clinical trial design and effector function assessment, alongside immune checkpoint inhibitors and tumor infiltrating lymphocytes, are key areas of focus for regulatory bodies. In the realm of manufacturing, the development of car structure designs, cell culture systems, and viral vector delivery methods are essential for large-scale production and cost-effective treatment options. Additionally, the integration of lymphocyte activation, minimal residual disease detection, and cytokine release syndrome management further enhances the therapeutic potential of car T-cell therapy.
- The car T-cell market's continuous unfolding is driven by the interplay of these evolving trends and the ongoing pursuit of innovation in immune effector cell-based therapies.
What are the Key Data Covered in this Car T-Cell Therapy Market Research and Growth Report?
-
What is the expected growth of the Car T-Cell Therapy Market between 2025 and 2029?
-
USD 3.56 billion, at a CAGR of 17.3%
-
-
What segmentation does the market report cover?
-
The report is segmented by End-user (Hospitals and Cancer treatment centers), Type (CD19, CD22, BCMA, and Others), and Geography (North America, Europe, Asia, and Rest of World (ROW))
-
-
Which regions are analyzed in the report?
-
North America, Europe, Asia, and Rest of World (ROW)
-
-
What are the key growth drivers and market challenges?
-
Growing awareness regarding CAR T-cell therapy, High cost associated with CAR T-cell therapy
-
-
Who are the major players in the Car T-Cell Therapy Market?
-
ACROBIOSYSTEMS INC., Allogene Therapeutics Inc., Bristol Myers Squibb Co., Celyad Oncology SA, Eli Lilly and Co., Fate Therapeutics Inc., Fortress Biotech Inc., Gilead Sciences Inc., GSK Plc, Johnson and Johnson Inc., Les Laboratoires Servier, Merck KGaA, Miltenyi Biotec B.V. and Co. KG, Noile Immune Biotech Inc., Novartis AG, Pfizer Inc., Sangamo Therapeutics Inc, and Sorrento Therapeutics Inc.
-
Market Research Insights
- The market is a dynamic and complex ecosystem, driven by ongoing research and innovation in the fields of immunotherapy, gene expression profiling, and protein engineering. According to recent estimates, The market is projected to reach USD15 billion by 2025, growing at a compound annual growth rate of 35%. This growth is fueled by the increasing demand for effective treatment options for various diseases, including cancer and autoimmune disorders. One key challenge in the market is toxicity management and treatment optimization. Intellectual property and access and affordability are also significant considerations, as the development and manufacturing of these therapies require substantial resources.
- Resistance mechanisms and disease relapse prevention are ongoing areas of research, with combination therapies and biomarker discovery playing crucial roles. Car T-cell therapy efficacy is a critical factor in market growth, with long-term outcomes and clinical trial results driving market momentum. In vivo imaging and immunotherapy development are essential components of the clinical translation process, while metabolic profiling and predictive modeling aid in the optimization of treatment regimens. Epigenetic modifications and cell signaling pathways are also under investigation for their potential to enhance car T-cell therapy efficacy. The market is further shaped by ongoing research in patient selection criteria, cell therapy manufacturing, preclinical development, and health economics.
We can help! Our analysts can customize this car t-cell therapy market research report to meet your requirements.