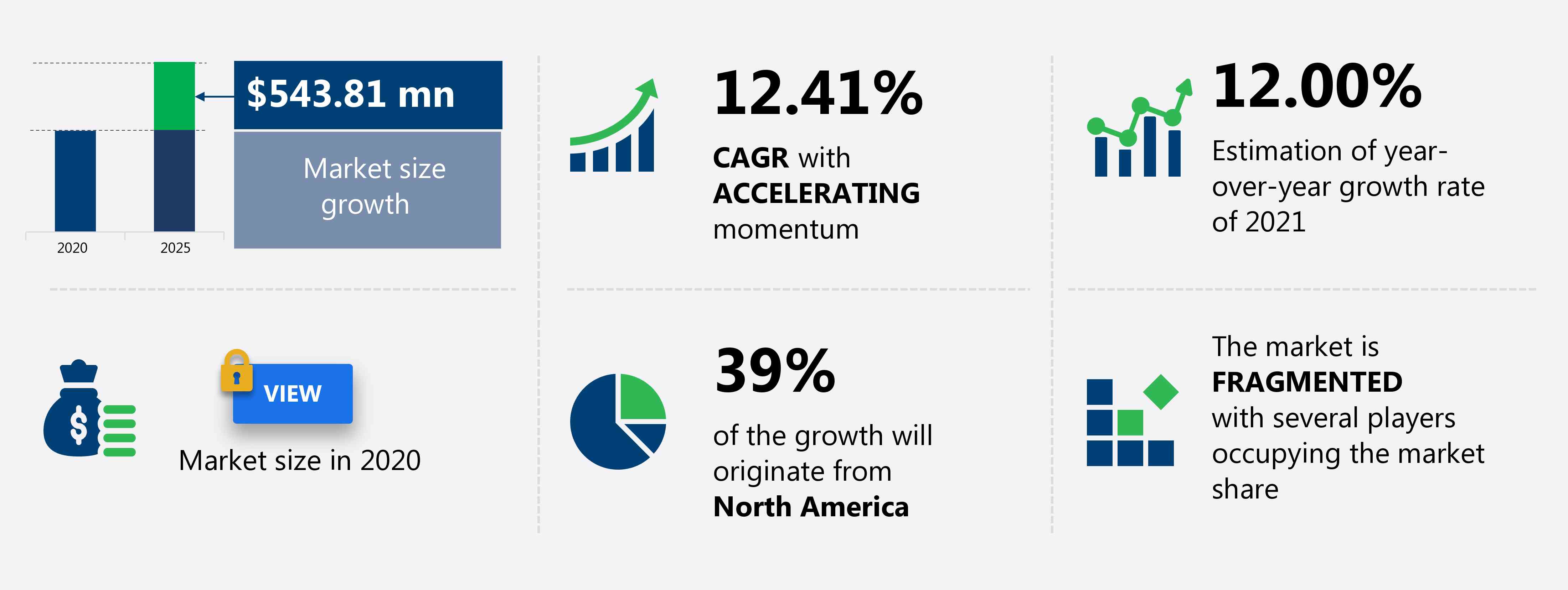
The drug eluting balloons market share is expected to increase by USD 543.81 million from 2020 to 2025, and the market’s growth momentum will accelerate at a CAGR of 12.41%.
This drug eluting balloons market research report provides valuable insights on the post-COVID-19 impact on the market, which will help companies evaluate their business approaches. Furthermore, this report extensively covers drug eluting balloons market segmentations by-product (peripheral DEBs and coronary DEBs) and geography (North America, Europe, Asia, and ROW). The drug eluting balloons market report also offers information on several market vendors, including B. Braun Melsungen AG, Becton Dickinson and Co., BIOTRONIK SE & Co. KG, Boston Scientific Corp., Cardinal Health Inc., Cardionovum GmbH, Cook Medical LLC, Koninklijke Philips NV, Medtronic Plc, and Opto Circuits (India) Ltd. among others.
What will the Drug-Eluting Balloons Market Size be During the Forecast Period?
Download the Free Report Sample to Unlock the Drug Eluting Balloons Market Size for the Forecast Period and Other Important Statistics
Drug Eluting Balloons Market: Key Drivers, Trends, and Challenges
Based on our research output, there has been a positive impact on the market growth during and post COVID-19 era. The growing demand for cath labs is notably driving the drug eluting balloons market growth, although factors such as stringent regulatory processes may impede the market growth. Our research analysts have studied the historical data and deduced the key market drivers and the COVID-19 pandemic impact on the drug eluting balloons industry. The holistic analysis of the drivers will help in deducing end goals and refining marketing strategies to gain a competitive edge.
Key Drug Eluting Balloons Market Driver
The growing demand for cath labs is one of the key factors driving the growth of the global drug eluting balloons market. Cath labs are using advanced technology such as flat panels that have superior image quality, which is developed by vendors such as GE Healthcare, Toshiba America Medical Systems, Siemens Medical Solutions, and Philips Medical Systems. Innova 2100 IQ and Innova 4100 systems by GE Healthcare are some of the other devices adopted in cath labs due to their improved image quality. These laboratories are often an integral part of hospitals, ASCs, and physicians' clinics and offer diagnostic services as out-patient procedures. Thus, the availability of cardiac catheterization and post-procedure recovery facilities within the same area can help minimize the overall associated expenses. In this way, the establishment of cath labs worldwide will increase the demand for DEBs globally. Such factors will increase the demand for the market in focus during the forecast period.
Key Drug Eluting Balloons Market Trend
The favorable reimbursement policies will fuel the global drug eluting balloons market growth. Advanced and innovative devices such as DEBs are more expensive than BMS and plain balloon catheters. End-users such as hospitals and ASCs will be reluctant to stock DEBs if reimbursement is not available. Earlier, DEBs were not covered under reimbursement, but since 2015, the reimbursement scenario has changed, and most insurance companies have started offering reimbursements for DEBs. In 2015, C.R Bard announced supplemental reimbursement policies for its Lutonix DEB after getting approval from Medicare and Medicaid services. The major reason for the supplemental reimbursement is to cover the additional cost to hospitals in the US for treating the beneficiaries with the Lutonix product in outpatient departments. In 2015, Medicare announced that the hospital inpatient cases, which use DEBs, are also eligible for the payment; this will help to cover the additional cost of DCBs. Also, Medtronic has been leading the efforts for reimbursements with its IN. PACT product, from Medicare, and the product got approval from new technology add-on payment (NTAP) for the treatment of PADs. So, favorable reimbursement policies will contribute to the growth of the market during the forecast period.
Key Drug Eluting Balloons Market Challenge
The stringent regulatory process is a major challenge for the global drug eluting balloons market growth. Product approvals and safety regulations are the biggest challenges for DEBs. Regulatory authorities have imposed a stringent regulatory framework and labeling requirement for DEBs as most of the devices are still under investigation and require abundant clinical data for regulatory approval. These regulations are often country-specific. DEBs are considered high-risk devices, classified as Class III medical devices, and have to undergo safety examination to demonstrate medical efficacy before being approved by regulatory agencies. The stringent policies hinder the entry of new companies into the market. In addition, DEBs need to undergo rigorous clinical testing and require premarket approval (PMA) for product approval. A PMA application is a data-driven decision, which depends on the safety and efficacy of the indicated patient population. The limited availability of clinical data is one of the reasons for the delayed approval of DEBs. Thus, the presence of stringent regulatory approvals is likely to hinder the growth prospects of the market.
This drug eluting balloons market analysis report also provides detailed information on other upcoming trends and challenges that will have a far-reaching effect on the market growth. The actionable insights on the trends and challenges will help companies evaluate and develop growth strategies for 2021-2025.
Parent Market Analysis
The global drug eluting balloons (DEBs) market is a part of the global healthcare equipment market. Our research report has extensively covered external factors influencing the parent market growth potential in the coming years, which will determine the levels of growth of the drug eluting balloons market during the forecast period.
Who are the Major Drug Eluting Balloons Market Vendors?
The report analyzes the market’s competitive landscape and offers information on several market vendors, including:
- B. Braun Melsungen AG
- Becton Dickinson and Co.
- BIOTRONIK SE & Co. KG
- Boston Scientific Corp.
- Cardinal Health Inc.
- Cardionovum GmbH
- Cook Medical LLC
- Koninklijke Philips NV
- Medtronic Plc
- Opto Circuits (India) Ltd.
This statistical study of the drug eluting balloons market encompasses successful business strategies deployed by the key vendors. The drug eluting balloons market is fragmented and the vendors are deploying organic and inorganic growth strategies to compete in the market.
Product Insights and News
- B. Braun Melsungen AG - The company offers DEBs such as SeQuent Please NEO, the next generation drug-coated balloon for the percutaneous transluminal coronary angioplasty.
To make the most of the opportunities and recover from the post-COVID-19 impact, market vendors should focus more on the growth prospects in the fast-growing segments, while maintaining their positions in the slow-growing segments.
The drug eluting balloons market forecast report offers in-depth insights into key vendor profiles. The profiles include information on the production, sustainability, and prospects of the leading companies.
Drug Eluting Balloons Market Value Chain Analysis
Our report provides extensive information on the value chain analysis for the drug eluting balloons market, which vendors can leverage to gain a competitive advantage during the forecast period. The end-to-end understanding of the value chain is essential in profit margin optimization and evaluation of business strategies. The data available in our value chain analysis segment can help vendors drive costs and enhance customer services during the forecast period.
The value chain of the global healthcare equipment market includes the following core components:
- Inputs
- Inbound logistics
- Operations
- Outbound logistics
- Marketing and sales
- Service
- Support activities
- Innovation
The report has further elucidated on other innovative approaches being followed by service providers to ensure a sustainable market presence.
Which are the Key Regions for Drug Eluting Balloons Market?
For more insights on the market share of various regions Request for a FREE sample now!
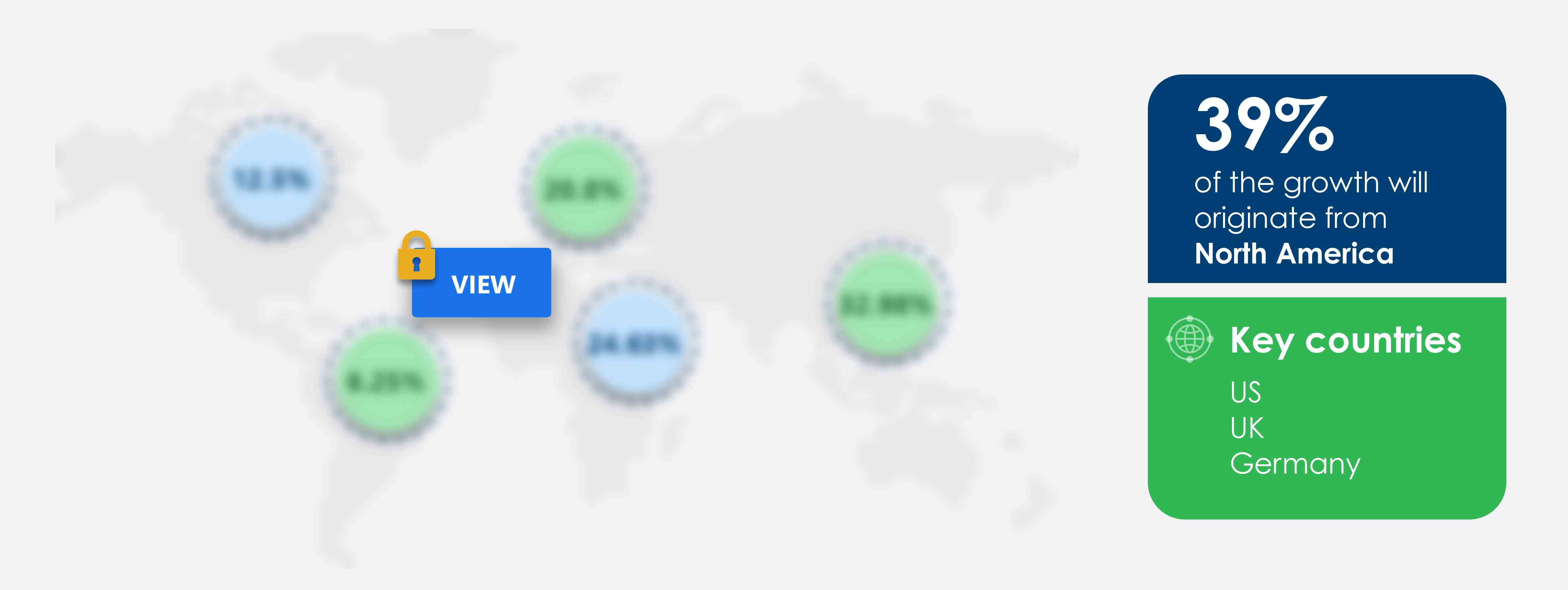
39% of the market’s growth will originate from North America during the forecast period. The US and Canada are the key markets for drug eluting balloons market in North America. Market growth in this region will be slower than the growth of the market in regions.
The growing demand for cath labs will facilitate the drug eluting balloons market growth in North America over the forecast period. This market research report entails detailed information on the competitive intelligence, marketing gaps, and regional opportunities in store for vendors, which will assist in creating efficient business plans.
COVID Impact and Recovery Analysis
In early 2020, the outbreak of COVID-19 in the region led to a halt of operations in manufacturing facilities, regulatory approvals, and vascular treatments, which restricted the production and use of DEBs and negatively impacted the growth of the market in the region. However, in 2021, the initiation of large-scale vaccination drives has lifted the lockdown and travel restrictions, which led to the resumption of supply chain activities. Such factors are expected to drive the market during the forecast period.
What are the Revenue-generating Product Segments in the Drug Eluting Balloons Market?
To gain further insights on the market contribution of various segments Request for a FREE sample
The drug-eluting balloons market share growth by the Peripheral DEBs segment will be significant during the forecast period. Peripheral DEBs are used for treating PADs, which are caused by the narrowing of the peripheral arteries in the stomach, legs, arms, and head and involve pain; cramping; and tiredness in the lower extremities, mainly in the legs while running, walking, and climbing stairs. People with PADs are at a higher risk of CADs, such as stroke, myocardial infarction, and stroke.
This report provides an accurate prediction of the contribution of all the segments to the growth of the drug eluting balloons market size and actionable market insights on the post-COVID-19 impact on each segment.
|
Drug Eluting Balloons Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
120 |
|
Base year |
2020 |
|
Forecast period |
2021-2025 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 12.41% |
|
Market growth 2021-2025 |
$ 543.81 million |
|
Market structure |
Fragmented |
|
YoY growth (%) |
12.00 |
|
Regional analysis |
North America, Europe, Asia, and ROW |
|
Performing market contribution |
North America at 39% |
|
Key consumer countries |
US, UK, Germany, Canada, and China |
|
Competitive landscape |
Leading companies, Competitive strategies, Consumer engagement scope |
|
Key companies profiled |
B. Braun Melsungen AG, Becton Dickinson and Co., BIOTRONIK SE & Co. KG, Boston Scientific Corp., Cardinal Health Inc., Cardionovum GmbH, Cook Medical LLC, Koninklijke Philips NV, Medtronic Plc, and Opto Circuits (India) Ltd. |
|
Market dynamics |
Parent market analysis, Market growth inducers and obstacles, Fast-growing and slow-growing segment analysis, COVID 19 impact and recovery analysis and future consumer dynamics, Market condition analysis for forecast period |
|
Customization purview |
If our report has not included the data that you are looking for, you can reach out to our analysts and get segments customized. |
What are the Key Data Covered in this Drug Eluting Balloons Market Report?
- CAGR of the market during the forecast period 2021-2025
- Detailed information on factors that will drive drug eluting balloons market growth during the next five years
- Precise estimation of the drug eluting balloons market size and its contribution to the parent market
- Accurate predictions on upcoming trends and changes in consumer behavior
- The growth of the drug eluting balloons industry across North America, Europe, Asia, and ROW
- A thorough analysis of the market’s competitive landscape and detailed information on vendors
- Comprehensive details of factors that will challenge the growth of drug eluting balloons market vendors
We can help! Our analysts can customize this report to meet your requirements. Get in touch
 Market_T.jpg?format=webp)