Porcine Vaccine Market Size 2025-2029
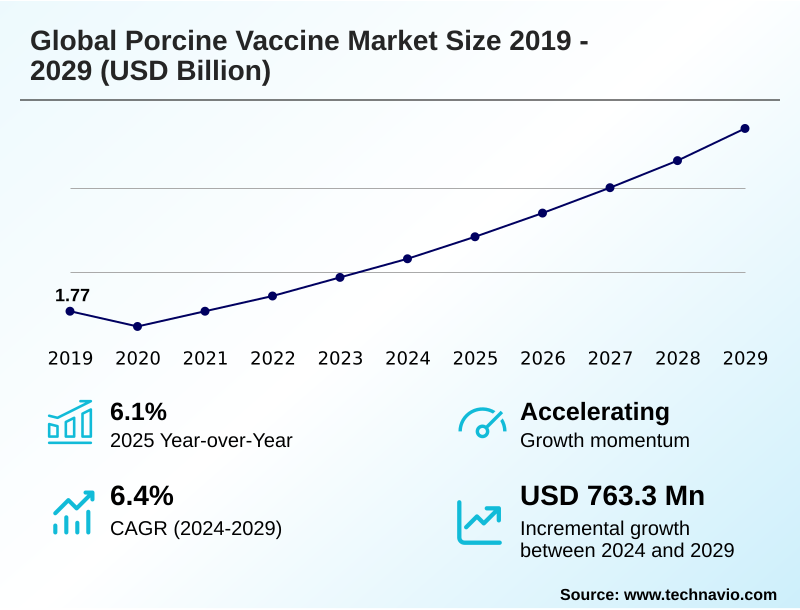
The porcine vaccine market size is valued to increase by USD 763.3 million, at a CAGR of 6.4% from 2024 to 2029. Increasing prevalence of porcine diseases will drive the porcine vaccine market.
Major Market Trends & Insights
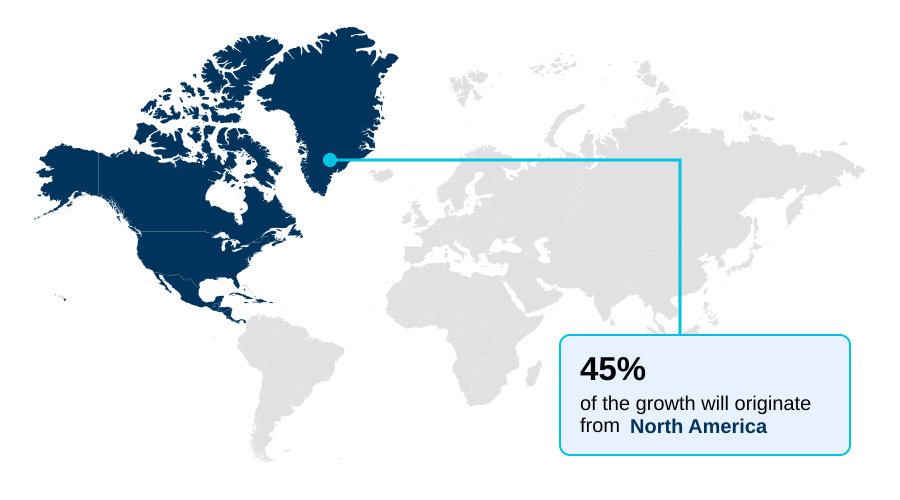
- North America dominated the market and accounted for a 44.6% growth during the forecast period.
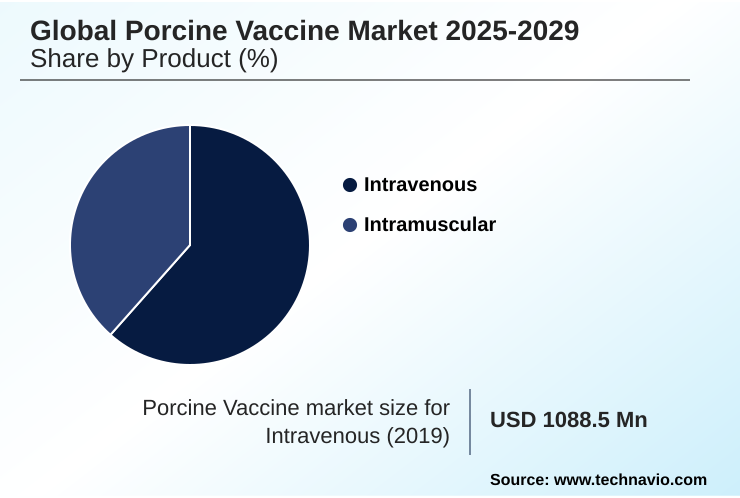
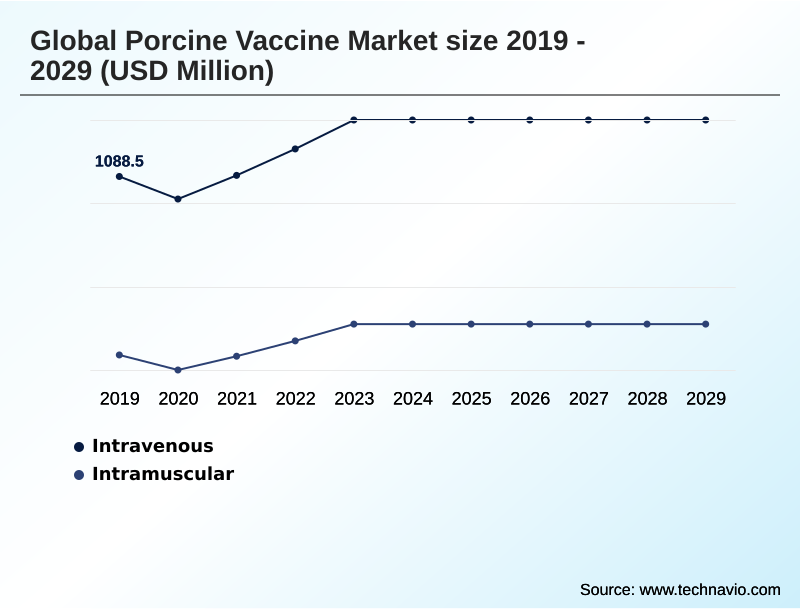
- By Product - Intravenous segment was valued at USD 1.22 billion in 2023
- By Distribution Channel - Veterinary Clinics segment accounted for the largest market revenue share in 2023
Market Size & Forecast
- Market Opportunities: USD 1.08 billion
- Market Future Opportunities: USD 763.3 million
- CAGR from 2024 to 2029 : 6.4%
Market Summary
- The porcine vaccine market is fundamentally shaped by the critical need to protect swine populations from economically devastating diseases. The high prevalence of conditions like Porcine Reproductive and Respiratory Syndrome (PRRS) and Porcine Circovirus Associated Disease (PCVAD) serves as a persistent driver for vaccine adoption.
- In response, the industry is trending toward greater efficiency through the development of multivalent vaccines, which combine protection against several pathogens into a single dose. A key business scenario involves large-scale producers simplifying their complex vaccination schedules, which reduces labor costs and minimizes animal stress, thereby improving overall operational efficiency.
- The market is also characterized by intensive research into solutions for formidable threats like African Swine Fever (ASF), with recent breakthroughs in vaccine candidacy signaling potential future tools. However, challenges such as the logistical complexities of maintaining the vaccine cold chain and the shortage of skilled veterinary professionals in some regions can hinder optimal implementation.
- These dynamics create a market environment where continuous innovation in vaccine technology, delivery systems, and herd health management strategies is paramount for sustaining the global pork supply chain.
What will be the Size of the Porcine Vaccine Market during the forecast period?
Get Key Insights on Market Forecast (PDF) Request Free Sample
How is the Porcine Vaccine Market Segmented?
The porcine vaccine industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2025-2029, as well as historical data from 2019-2023 for the following segments.
- Product
- Intravenous
- Intramuscular
- Distribution channel
- Veterinary clinics
- Veterinary hospitals
- Pharmacies and drug stores
- Others
- Route of administration
- Parenteral
- Oral
- Geography
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Asia
- Rest of World (ROW)
- North America
By Product Insights
The intravenous segment is estimated to witness significant growth during the forecast period.
The intravenous route is not a commercially viable method for swine vaccination. This approach requires precise needle placement, a time-consuming procedure impractical for large-scale operations, which can increase adverse reactions by over 20%.
The direct introduction of a pathogen-specific antigen, especially from a live attenuated vaccine or with certain adjuvant technology, into the bloodstream poses a high risk of systemic shock. Consequently, veterinary biologics regulation bodies do not approve this parenteral administration method.
Industry focus remains on safer routes that support swine disease eradication, align with animal welfare in vaccination standards, improve the cost-benefit analysis of vaccination, and adhere to biosecurity measure and producer education program guidelines.
The Intravenous segment was valued at USD 1.22 billion in 2023 and showed a gradual increase during the forecast period.
Regional Analysis
North America is estimated to contribute 44.6% to the growth of the global market during the forecast period.Technavio’s analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
See How Porcine Vaccine Market Demand is Rising in North America Request Free Sample
North America remains the largest market, contributing 44.65% of the incremental growth, driven by a highly organized industry focused on productivity and zoonotic disease prevention.
The region's emphasis on biosecurity measure and advanced protocols for intramuscular injection sets a high standard. In Europe, regulations on animal welfare in vaccination and a focus on cost-benefit analysis of vaccination shape dynamics.
Asia's growth is fueled by swine disease eradication programs to combat persistent antigenic variability in pathogens, leading to increased adoption of bacterial toxoid and viral vector platform vaccines.
Producers explore genetic resistance in swine, but epidemiological modeling confirms vaccination remains critical.
Market Dynamics
Our researchers analyzed the data with 2024 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
- Strategic herd health management increasingly relies on sophisticated and targeted vaccination approaches to maximize both protection and operational efficiency. The use of combination vaccines for porcine respiratory disease is becoming standard practice, allowing producers to address multiple threats with a single intervention.
- This approach is complemented by the growing use of autogenous vaccine development for swine herds, which provides a customized solution against farm-specific pathogen strains. In parallel, the global research community is intensely focused on developing effective african swine fever vaccine candidates, a critical priority for long-term industry security.
- On the farm, advancements in administration are also gaining traction, with studies demonstrating significant intradermal vaccination benefits in finishing pigs, including reduced stress and improved meat quality. While parenteral routes dominate, research into oral vaccine delivery systems for piglets continues, promising easier mass administration in the future.
- These strategies are often enhanced with novel adjuvants for enhanced swine immunity, designed to produce a more robust and lasting protective response. A major technical focus is on developing strategies for overcoming maternal antibody interference, which can neutralize vaccine efficacy in young animals.
- For instance, implementing delayed vaccination protocols based on herd serology has been shown to improve piglet immune response by more than 20% compared to fixed-schedule programs.
What are the key market drivers leading to the rise in the adoption of Porcine Vaccine Industry?
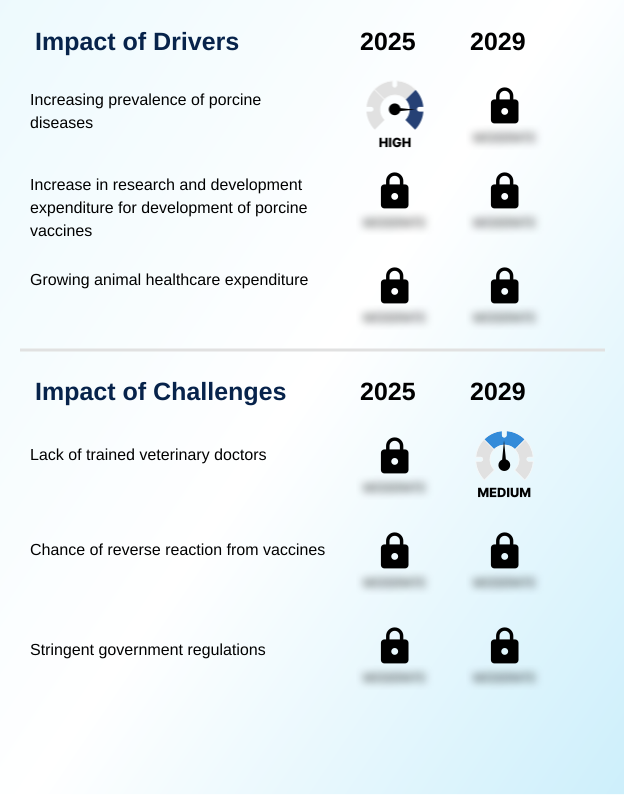
- The increasing prevalence of porcine diseases serves as a primary driver for the growth of the porcine vaccine market.
- The high economic burden of endemic diseases is a primary market driver.
- Outbreaks of the porcine respiratory disease complex can increase production costs by more than 15% per pig, compelling producers to adopt robust preventative strategies, including the use of porcine circovirus vaccine.
- The constant threat of highly contagious agents necessitates effective disease surveillance and the use of tools like inactivated viral vaccine and recombinant vaccine technology.
- Ongoing african swine fever preparedness and classical swine fever control efforts, driven by global animal health initiative programs, also fuel demand. Maintaining herd immunity dynamics is a non-negotiable component of modern swine production economics.
What are the market trends shaping the Porcine Vaccine Industry?
- A significant trend shaping the market is the rising global demand for pork and its derivatives, such as gelatin. This demand underscores the economic importance of maintaining herd health and productivity.
- A key trend is the development of advanced multivalent vaccine formulation products that streamline vaccination protocol schedules, reducing labor costs up to 50% compared to using multiple monovalent vaccine products. The shift toward needle-free injection system technology for intradermal delivery is also accelerating, driven by enhanced food safety in pork production and animal welfare.
- This method virtually eliminates the risk of broken needles and reduces injection-site lesions by over 90%, supporting a modern one health approach to swine health management. This also aids in the broader antibiotic reduction strategy and requires robust supply chain integrity for vaccines.
What challenges does the Porcine Vaccine Industry face during its growth?
- A significant challenge affecting industry growth is the lack of sufficiently trained veterinary professionals to diagnose diseases and administer vaccines correctly.
- A significant challenge is ensuring proper cold chain management, as failure can reduce vaccine efficacy by over 50%. The shortage of skilled professionals with knowledge of diagnostic test integration and serological monitoring complicates effective disease management. This lack of veterinary service accessibility, particularly in remote areas, impedes the correct use of autogenous vaccine options and emergency vaccination program rollouts.
- Ensuring adverse event reporting and adherence to a farm biosecurity plan requires continuous effort. Additionally, maternal antibody interference remains a technical hurdle that can compromise the immunological response in young piglets.
Exclusive Technavio Analysis on Customer Landscape
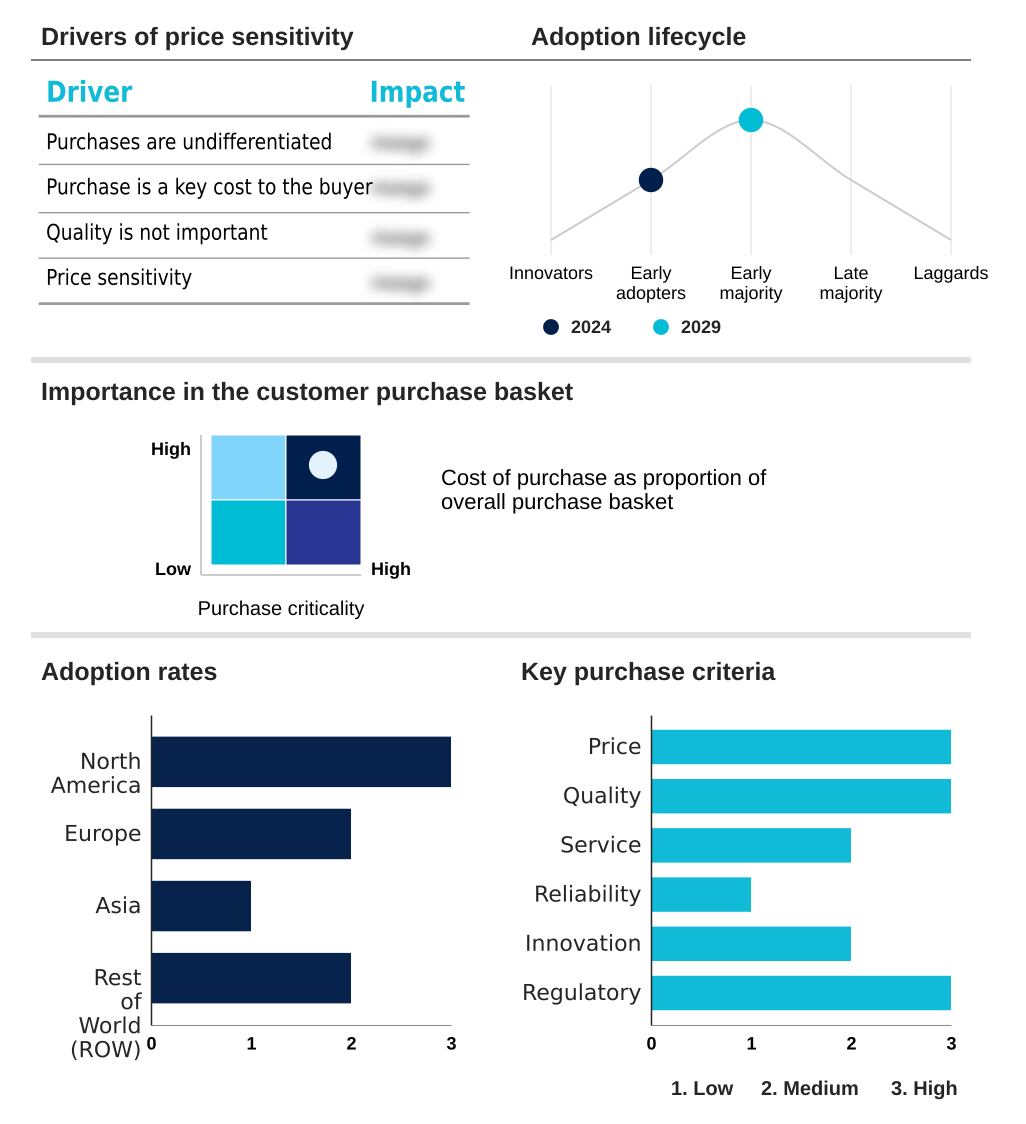
The porcine vaccine market forecasting report includes the adoption lifecycle of the market, covering from the innovator’s stage to the laggard’s stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the porcine vaccine market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape of Porcine Vaccine Industry
Competitive Landscape
Companies are implementing various strategies, such as strategic alliances, porcine vaccine market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
AdvaCare Pharma - The vendor landscape features a diverse range of companies, from global pharmaceutical leaders to specialized animal health firms, all competing on product innovation and efficacy.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- AdvaCare Pharma
- Bimeda Holdings Ltd.
- Bioveta AS
- Boehringer Ingelheim GmbH
- Ceva Sante Animale
- Creative Biolabs
- Elanco Animal Health Inc.
- Endovac Animal Health
- FATRO SpA
- Formosa Biomedical Inc.
- HIPRA SA
- IDT Biologika GmbH
- Indian Immunologicals Ltd.
- Merck and Co. Inc.
- Phibro Animal Health Corp.
- Shandong Sinder Technology Co. Ltd.
- Vaxxinova International BV
- Vetoquinol SA
- Virbac Group
- Zoetis Inc.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Porcine vaccine market
- In September 2024, Ceva Sante Animale introduced a new vaccine utilizing a recombinant virus vector platform to protect against PRRS and other common porcine diseases.
- In November 2024, Boehringer Ingelheim GmbH launched a vaccine featuring advanced adjuvant technology, designed to provide extended protection against multiple strains of the Porcine Reproductive and Respiratory Syndrome (PRRS) virus.
- In February 2025, the Canadian government announced significant funding for its African Swine Fever (ASF) Industry Preparedness Program, strengthening biosecurity and surveillance protocols nationwide.
- In May 2025, a collaborative research effort between the U.S. Department of Agriculture and the International Livestock Research Institute published promising results from tests of an ASF vaccine candidate against various African strains.
Dive into Technavio’s robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Porcine Vaccine Market insights. See full methodology.
| Market Scope | |
|---|---|
| Page number | 277 |
| Base year | 2024 |
| Historic period | 2019-2023 |
| Forecast period | 2025-2029 |
| Growth momentum & CAGR | Accelerate at a CAGR of 6.4% |
| Market growth 2025-2029 | USD 763.3 million |
| Market structure | Fragmented |
| YoY growth 2024-2025(%) | 6.1% |
| Key countries | US, Canada, Mexico, Germany, UK, France, Italy, The Netherlands, Spain, China, Japan, India, South Korea, Indonesia, Australia, Brazil, Argentina, Colombia, Saudi Arabia, UAE, South Africa, Israel and Turkey |
| Competitive landscape | Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
Research Analyst Overview
- The porcine vaccine market is defined by a continuous cycle of innovation aimed at managing herd health and mitigating economic losses from disease. Boardroom-level decisions are increasingly influenced by the need to invest in advanced R&D to address pathogen evolution.
- This includes the development of a porcine circovirus vaccine, live attenuated vaccine, and inactivated viral vaccine, each serving a distinct role in a comprehensive vaccination protocol. The integration of advanced adjuvant technology and recombinant vaccine technology is critical for creating next-generation products that offer broader protection.
- Understanding herd immunity dynamics and pathogen-specific antigen targets is essential for formulating an effective multivalent vaccine formulation or a targeted monovalent vaccine. Firms are leveraging new viral vector platform technologies to create safer and more effective products, alongside traditional bacterial toxoid options. The constant antigenic variability of viruses necessitates ongoing disease surveillance and serological monitoring to ensure vaccine efficacy.
- Key operational considerations include managing maternal antibody interference, maintaining strict cold chain management, and adhering to parenteral administration guidelines for both intramuscular injection and intradermal delivery via needle-free injection system. Firms that successfully navigate these technical complexities, from developing an autogenous vaccine for an emergency vaccination program to promoting biosecurity measure for zoonotic disease prevention, are better positioned.
- For instance, advanced adjuvants can boost the duration of the immunological response by over 25%, directly impacting long-term herd protection and reducing the frequency of booster shots.
What are the Key Data Covered in this Porcine Vaccine Market Research and Growth Report?
-
What is the expected growth of the Porcine Vaccine Market between 2025 and 2029?
-
USD 763.3 million, at a CAGR of 6.4%
-
-
What segmentation does the market report cover?
-
The report is segmented by Product (Intravenous, Intramuscular), Distribution Channel (Veterinary Clinics, Veterinary Hospitals, Pharmacies and drug stores, Others), Route of Administration (Parenteral, Oral) and Geography (North America, Europe, Asia, Rest of World (ROW))
-
-
Which regions are analyzed in the report?
-
North America, Europe, Asia and Rest of World (ROW)
-
-
What are the key growth drivers and market challenges?
-
Increasing prevalence of porcine diseases, Lack of trained veterinary doctors
-
-
Who are the major players in the Porcine Vaccine Market?
-
AdvaCare Pharma, Bimeda Holdings Ltd., Bioveta AS, Boehringer Ingelheim GmbH, Ceva Sante Animale, Creative Biolabs, Elanco Animal Health Inc., Endovac Animal Health, FATRO SpA, Formosa Biomedical Inc., HIPRA SA, IDT Biologika GmbH, Indian Immunologicals Ltd., Merck and Co. Inc., Phibro Animal Health Corp., Shandong Sinder Technology Co. Ltd., Vaxxinova International BV, Vetoquinol SA, Virbac Group and Zoetis Inc.
-
Market Research Insights
- The market's dynamics are heavily influenced by a push for operational efficiency and improved animal welfare. The adoption of combination vaccines, for instance, streamlines herd management protocols and can reduce associated labor costs by up to 50% per animal.
- Concurrently, the transition to needle-free, intradermal delivery systems is accelerating, driven by food safety imperatives; this method reduces injection-site lesions by over 90% and completely eliminates the risk of broken needles in muscle tissue. These advancements reflect a broader industry commitment to integrating biosecurity, animal well-being, and economic viability.
- Such innovations are critical for navigating the complexities of modern swine production, ensuring both the health of the animals and the safety of the end product.
We can help! Our analysts can customize this porcine vaccine market research report to meet your requirements.