Anti-Thrombin III Testing Market Size 2024-2028
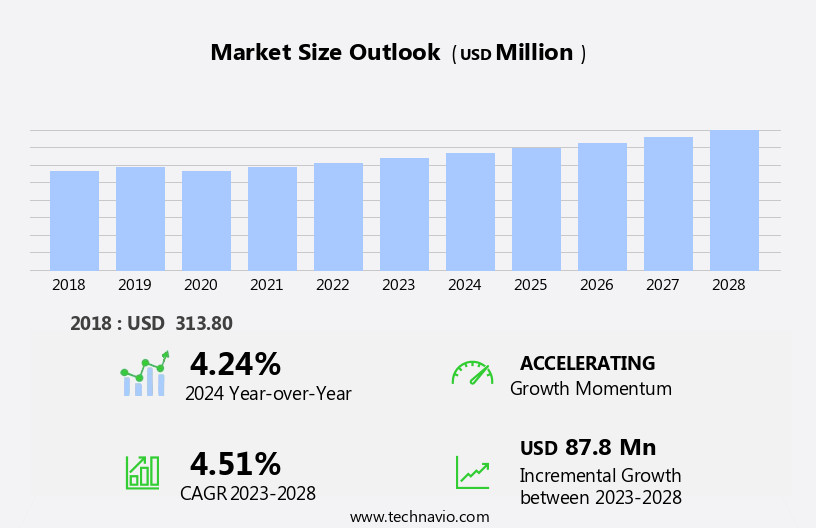
The anti-thrombin iii testing market size is forecast to increase by USD 87.8 million at a CAGR of 4.51% between 2023 and 2028.
- The market is witnessing significant growth due to the existence of various tests to detect anti-thrombin III deficiency. This is particularly driven by the increasing prevalence of blood clotting disorders, which necessitate regular monitoring of anti-thrombin III levels. However, the lack of clinically available protocols to treat anti-thrombin deficiency poses a challenge to market growth. This market analysis report provides an in-depth examination of these trends and the impact they have on the market. The presence of numerous blood clotting disorders, such as deep vein thrombosis and pulmonary embolism, necessitates regular testing for anti-thrombin III levels. The absence of effective treatment options for anti-thrombin deficiency, however, limits the market's growth potential.Despite this challenge, the market is expected to witness steady growth due to the increasing awareness and diagnosis of blood clotting disorders. The report offers a comprehensive analysis of the market trends, growth drivers, and challenges, providing valuable insights for stakeholders.
What will be the Size of the Anti-Thrombin III Testing Market During the Forecast Period?
- The anti-thrombin III (AT III) testing market encompasses diagnostic solutions for detecting deficiencies and imbalances of this essential protein In the blood, which plays a critical role in inhibiting blood clotting. The market is driven by the increasing prevalence of thrombosis-related conditions, such as deep vein thrombosis (DVT) and hereditary antithrombin deficiency (ATD), fueling demand for accurate and reliable testing methods. Major market segments include therapeutics, where AT III is used to treat various clotting disorders, and research institutes, hospitals, and clinics for diagnostic purposes. Technologies employed in AT III testing include activity assays and immunological assays, with the former measuring the functional activity of the protein and the latter detecting the presence of AT III in a blood sample.
- Advancements in alternative names, such as functional antithrombin III and AT III activity, and the emergence of point-of-care testing, artificial intelligence, and large molecular weight heparin (LMWH) In the market, contribute to its growth. The market is further influenced by the ongoing research in personalized medicine and the development of new medicines to address various thrombosis-related conditions. Overall, the AT III testing market is poised for significant expansion due to the increasing focus on early diagnosis and effective management of blood clotting disorders.
How is this Anti-Thrombin III Testing Industry segmented and which is the largest segment?
The anti-thrombin iii testing industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2024-2028, as well as historical data from 2018-2022 for the following segments.
- End-user
- Hospitals
- Laboratories
- Academic and research institutes
- Geography
- North America
- Canada
- US
- Europe
- Germany
- UK
- Asia
- China
- Rest of World (ROW)
- North America
By End-user Insights
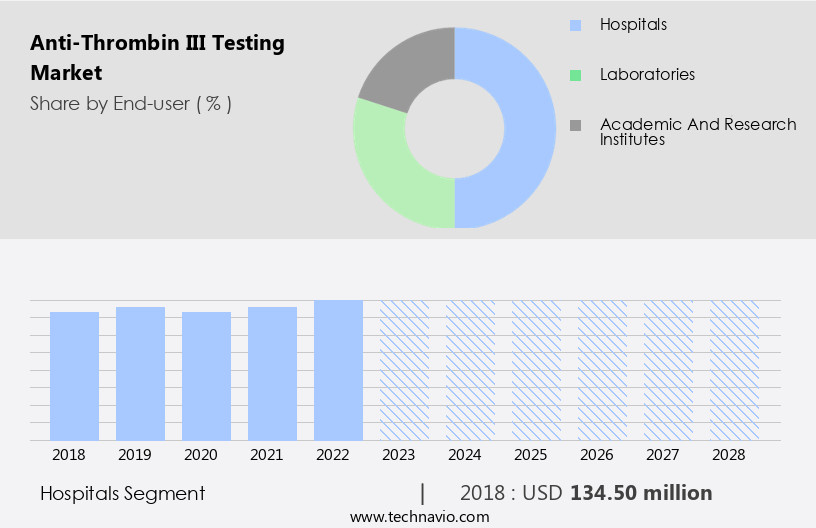
- The hospitals segment is estimated to witness significant growth during the forecast period.
Hospitals, research institutes, and clinics utilize anti-thrombin III testing products for diagnosing and managing various conditions, particularly cardiovascular diseases and thrombosis. These testing products include assay kits, reagents, and systems used for activity and immunological assays. Hospitals, such as Seattle Children's Hospital, perform these tests to determine antithrombin III levels in patients with suspected hereditary antithrombin deficiency, AT III deficiency, or thrombosis. Thrombosis management is crucial for preventing complications like deep vein thrombosis, pulmonary embolism, and thrombus formation. The increasing prevalence of conditions associated with thrombosis, such as liver failure, kidney disease, spreading cancer, and metastatic disease, necessitates the use of anti-thrombin III testing.
Additionally, advancements in technology, such as point-of-care testing and personalized medicine, contribute to the growing demand for these tests. Key genes related to antithrombin production, such as SERPINC1, are also under investigation using artificial intelligence and advanced analytics.
Get a glance at the Anti-Thrombin III Testing Industry report of share of various segments Request Free Sample
The Hospitals segment was valued at USD 134.50 million in 2018 and showed a gradual increase during the forecast period.
Regional Analysis
- North America is estimated to contribute 46% to the growth of the global market during the forecast period.
Technavio's analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
For more insights on the market share of various regions, Request Free Sample
The global anti-thrombin III (AT III) testing market is significantly driven by the increasing prevalence of thrombosis-related conditions, such as deep vein thrombosis (DVT) and pulmonary embolism (PE), which require accurate and timely diagnosis. According to Acta Haematologica, hereditary antithrombin deficiency and AT III deficiency are among the leading causes of thrombosis. The British Heart Foundation reports that these conditions affect approximately 1 in 5,000 people. The therapeutics segment, which includes plasma-derived antithrombin concentrate (AT III) and synthetic anticoagulants, dominates the market due to the high demand for these products in thrombosis management. The use of antithrombin concentrates is particularly important in cases of hereditary antithrombin deficiency, where the body naturally produces insufficient amounts of the protein.
Advancements in technology have led to the development of alternative testing methods, such as activity assays and immunological assays, which offer faster and more accurate results compared to traditional methods. Point-of-care testing is gaining popularity due to its convenience and ability to provide results in real-time, enabling personalized medicine and improved thrombosis management. Major players In the market include Octapharma, Atenative, and others. The market is expected to grow due to the increasing incidence of cardiovascular diseases, the need for early diagnosis, and the development of new technologies. However, challenges such as excessive bleeding, fainting, hematoma, infection, and the high cost of testing may hinder market growth.
Keywords: Anti-thrombin III testing, Thrombosis, Blood clotting, Blood test, Cardiovascular diseases, Thrombosis management, Protein, Plasma-derived antithrombin concentrate, Hereditary antithrombin deficiency, AT III deficiency, DVT, Pulmonary embolism, Activity assays, Immunological assays, Point-of-care testing, Personalized medicine.
Market Dynamics
Our researchers analyzed the data with 2023 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
What are the key market drivers leading to the rise In the adoption of Anti-Thrombin III Testing Industry?
Existence of tests related to anti-thrombin III is the key driver of the market.
- Anti-Thrombin III testing plays a crucial role in identifying individuals with hereditary antithrombin deficiency, a condition characterized by insufficient levels of this protein, leading to an increased risk of thrombosis in veins and arteries. This testing encompasses various methods, including activity assays and immunological assays, to determine the presence and functionality of Anti-Thrombin III (AT III) In the blood. Acta Haematologica and Thrombosis Update are notable publications that have featured extensive research on AT III deficiency and its diagnosis. The British Heart Foundation and various research institutes and clinics have also contributed significantly to the understanding of this condition.
- Plasma-derived antithrombin concentrate, such as Octapharma's Atenative, is used therapeutically to prevent and manage thrombosis in patients with AT III deficiency. LMWH (low molecular weight heparin) and NSO (Nadroparin sodium) are also commonly used anticoagulants for thrombosis management. Functional antithrombin III testing is essential for diagnosing AT III deficiency, which can lead to deep vein thrombosis (DVT) and pulmonary embolism. Abnormal test results may indicate an increased risk of excessive bleeding, fainting, hematoma, infection, or thrombosis. Antithrombin III testing is not limited to adults; it is also crucial for newborns, infants, and adults with liver failure, kidney disease, spreading cancer, metastatic disease, protein C deficiency, protein S deficiency, and Factor V Leiden.
- Point-of-care testing and personalized medicine are emerging trends in thrombosis management, allowing for quicker and more accurate diagnosis and treatment. The use of artificial intelligence and advanced techniques, such as activity assays and immunological assays, can enhance the accuracy and efficiency of AT III testing. The normal ranges for AT III antigen and activity levels vary depending on the age and health status of the individual. Abnormal results may require further investigation and consultation with a healthcare provider. It is important to note that AT III testing involves a blood sample collection using a needle, which may cause pain, bruising, or discomfort.
- However, the benefits of early diagnosis and appropriate treatment far outweigh the minor discomfort associated with the test.
What are the market trends shaping the Anti-Thrombin III Testing Industry?
Presence of various blood clotting disorders is the upcoming market trend.
- Anti-thrombin III (AT III) testing plays a crucial role in diagnosing and managing various thrombotic disorders, including hereditary antithrombin deficiency and AT III deficiency. Acta Haematologica and Thrombosis Update are renowned journals publishing research on these conditions. Plasma-derived antithrombin concentrate, such as Octapharma's Atenative, is used for therapeutic purposes. Symptoms of AT deficiency, including deep vein thrombosis (DVT) and pulmonary embolism, can be mistaken for other conditions, like protein C deficiency or protein S deficiency. Protein C deficiency, an inherited coagulation disorder, results in approximately 50% of the normal level of this protein In the blood, leading to the formation of blood clots and pulmonary emboli.
- Symptoms include DVT, pulmonary embolism, and thrombosis In the venous sinuses of the brain. Protein S deficiency, another inherited disorder, is characterized by the recurrent formation of blood clots and emboli. Both protein C and S deficiencies can lead to excessive bleeding, fainting, hematoma, infection, and abnormal test results. Diagnostic tests for these conditions include antigen tests, activity assays, and immunological assays. These tests involve taking a blood sample, which may cause pain, bruising, or the risk of infection. Advancements in technology have led to point-of-care testing and personalized medicine, making thrombosis management more efficient. Artificial intelligence and functional antithrombin III tests are also being explored for their potential in diagnosing and managing thrombotic disorders.
- Conditions like liver failure, kidney disease, spreading cancer, and metastatic disease can also lead to AT deficiencies. It is essential for healthcare providers to be aware of these underlying conditions and the importance of proper AT III testing to ensure accurate diagnoses and effective treatment plans. Normal ranges for AT III levels vary for newborns, infants, and adults. Abnormal results may indicate an increased risk for thrombosis or bleeding problems. It is crucial for patients to discuss any concerns or symptoms with their healthcare provider to determine the appropriate course of action.
What challenges does the Anti-Thrombin III Testing Industry face during its growth?
Lack of clinically available protocols to treat anti-thrombin deficiency is a key challenge affecting the industry growth.
- Anti-Thrombin III (AT III) testing plays a crucial role in diagnosing and managing various thrombotic conditions, including hereditary antithrombin deficiency and AT III deficiency. Acta Haematologica, Thrombosis Update, and Frontiers in Neurology are leading journals publishing research on this topic. Plasma-derived antithrombin concentrates, such as Octapharma's Atenative, are essential therapeutics for treating these conditions. Hematologists use these concentrates during surgeries, neuro-surgeries, severe trauma, and child delivery to prevent venous clots when blood thinners like Low Molecular Weight Heparin (LMWH) and Normal Dose Heparin (NSO) are contraindicated due to the risk of excessive bleeding. Functional AT III activity assays and immunological antigen tests are used to diagnose AT deficiencies.
- These tests are crucial for identifying patients with heparin resistance, who require the administration of antithrombin concentrates to achieve effective thrombosis management. Conditions like deep vein thrombosis (DVT), pulmonary embolism, liver failure, kidney disease, spreading cancer, metastatic disease, protein C deficiency, protein S deficiency, and Factor V Leiden can lead to AT deficiencies. Antithrombin III testing is a blood test that measures the protein's activity and antigen levels in a blood sample. Abnormal test results may indicate an increased risk of thrombosis or bleeding problems. Patients may experience symptoms such as pain, bruising, fainting, hematoma, infection, or excessive bleeding.
- It is essential for healthcare providers to consider these risks when interpreting test results and administering treatments. Point-of-care testing and personalized medicine are emerging trends in AT III testing, allowing for faster and more accurate results. These advancements can lead to improved thrombosis management and better patient outcomes. However, further research is required to establish standardized normal ranges for different populations, including newborns, infants, and adults.
Exclusive Customer Landscape
The anti-thrombin iii testing market forecasting report includes the adoption lifecycle of the market, covering from the innovator's stage to the laggard's stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the anti-thrombin iii testing market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape
Key Companies & Market Insights
Companies are implementing various strategies, such as strategic alliances, anti-thrombin iii testing market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence In the industry.
Abbott Laboratories - The market encompasses diagnostic solutions that measure the level of this essential anticoagulant In the blood. Anti-thrombin III plays a crucial role in preventing blood clots, making accurate testing essential for monitoring anticoagulation therapy. One company specializes in providing comprehensive diagnostic tools for this purpose, including the CP3000 lab auto system assembly kit. This product facilitates efficient and reliable anti-thrombin III testing, ensuring precise results for healthcare professionals.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Abbott Laboratories
- Beckman Coulter Inc.
- Becton Dickinson and Co.
- Bio Rad Laboratories Inc.
- BIRON HEALTH GROUP
- Danaher Corp.
- F. Hoffmann La Roche Ltd.
- Grifols SA
- Invitae Corp.
- Merck KGaA
- Meridian Bioscience Inc.
- Oy Medix Biochemica Ab
- Randox Laboratories Ltd.
- Scripps Laboratories Inc.
- Sekisui Diagnostics LLC
- Siemens AG
- Sysmex Corp.
- Thermo Fisher Scientific Inc.
- Transasia Bio Medicals Ltd.
- Werfenlife SA
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Research Analyst Overview
The anti-thrombin III (AT III) testing market encompasses the diagnostic assessment of AT III levels and functions In the context of various clotting disorders and diseases. This market caters to the demand for accurate and reliable testing solutions to aid healthcare providers in effective thrombosis management. AT III is a vital protein In the blood that plays a significant role in preventing excessive blood clotting. Its deficiency can lead to various thrombotic conditions, including deep vein thrombosis (DVT) and pulmonary embolism (PE). Consequently, the importance of AT III testing lies In the early identification and diagnosis of AT III deficiencies, enabling timely intervention and treatment.
The market for AT III testing is driven by the increasing prevalence of thrombotic disorders and the growing need for personalized medicine. Thrombosis management has emerged as a critical area of focus in healthcare, with a shift towards point-of-care testing (POCT) to facilitate faster and more convenient diagnosis. Two primary types of AT III tests are available: antigen tests and activity assays. Antigen tests measure the quantity of AT III present in a blood sample, while activity assays determine the functional capacity of AT III to inhibit thrombin. Both tests are essential In the diagnosis and monitoring of AT III deficiencies.
The use of alternative names for AT III, such as antithrombin or antithrombin III, should not confuse the market analysis. AT III deficiencies can be hereditary or acquired, with various underlying causes, including liver failure, kidney disease, spreading cancer, metastatic disease, protein C deficiency, protein S deficiency, and factor V Leiden mutation. AT III testing is crucial for various patient populations, including newborns, infants, and adults. Abnormal test results can indicate an increased risk for thrombotic events, such as DVT, PE, and bleeding problems. These risks can manifest as symptoms like pain, bruising, excessive bleeding, fainting, hematoma, infection, and the formation of blood clots or thrombi in veins and arteries.
The market for AT III testing is expected to grow significantly due to the increasing demand for accurate and reliable testing solutions. The ongoing advancements in technology, such as the integration of artificial intelligence (AI) in testing and the development of new chemicals and assays, are further expected to drive market growth. In conclusion, the market represents a vital segment In the healthcare industry, catering to the growing demand for accurate and reliable testing solutions to aid In the diagnosis and management of thrombotic disorders. The market is driven by the increasing prevalence of thrombotic disorders, the need for personalized medicine, and the ongoing technological advancements in testing.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
153 |
|
Base year |
2023 |
|
Historic period |
2018-2022 |
|
Forecast period |
2024-2028 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 4.51% |
|
Market growth 2024-2028 |
USD 87.8 million |
|
Market structure |
Fragmented |
|
YoY growth 2023-2024(%) |
4.24 |
|
Key countries |
US, Germany, China, UK, and Canada |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
What are the Key Data Covered in this Anti-Thrombin III Testing Market Research and Growth Report?
- CAGR of the Anti-Thrombin III Testing industry during the forecast period
- Detailed information on factors that will drive the growth and forecasting between 2024 and 2028
- Precise estimation of the size of the market and its contribution of the industry in focus to the parent market
- Accurate predictions about upcoming growth and trends and changes in consumer behaviour
- Growth of the market across North America, Europe, Asia, and Rest of World (ROW)
- Thorough analysis of the market's competitive landscape and detailed information about companies
- Comprehensive analysis of factors that will challenge the anti-thrombin iii testing market growth of industry companies
We can help! Our analysts can customize this anti-thrombin iii testing market research report to meet your requirements.