Endovascular Abdominal Aortic Aneurysm (AAA) Repair Devices Market Size 2025-2029
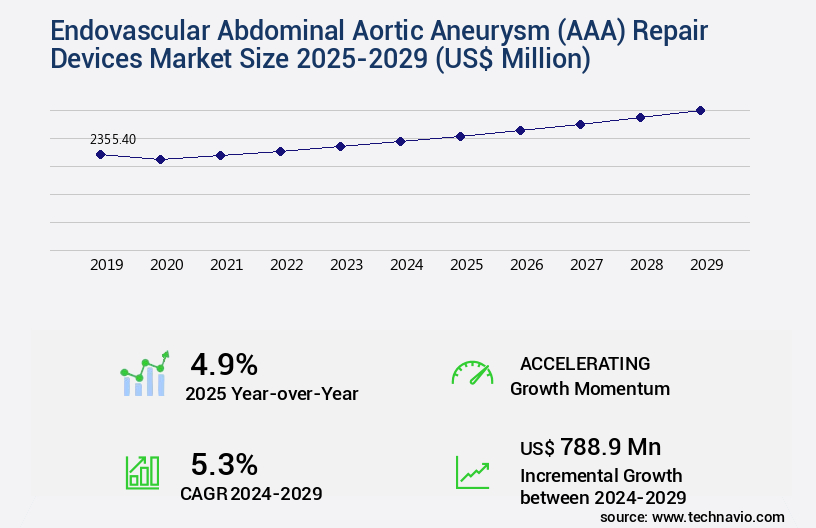
The endovascular abdominal aortic aneurysm (AAA) repair devices market size is valued to increase by USD 788.9 million, at a CAGR of 5.3% from 2024 to 2029. Aging population and rising prevalence of abdominal aortic aneurysms will drive the endovascular abdominal aortic aneurysm (AAA) repair devices market.
Market Insights
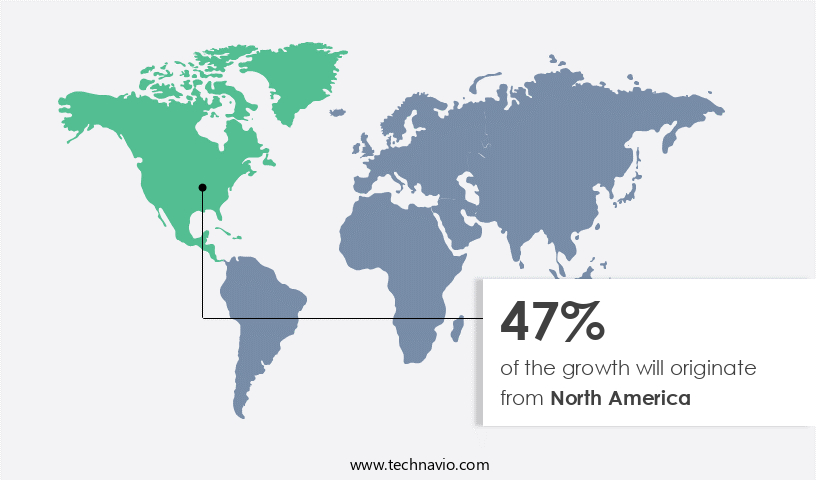
- North America dominated the market and accounted for a 47% growth during the 2025-2029.
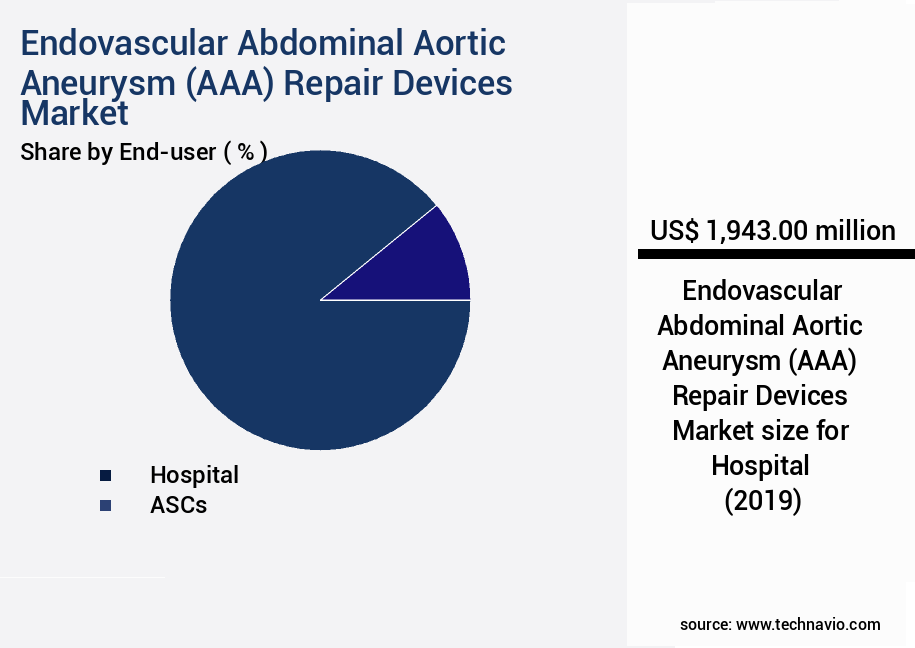
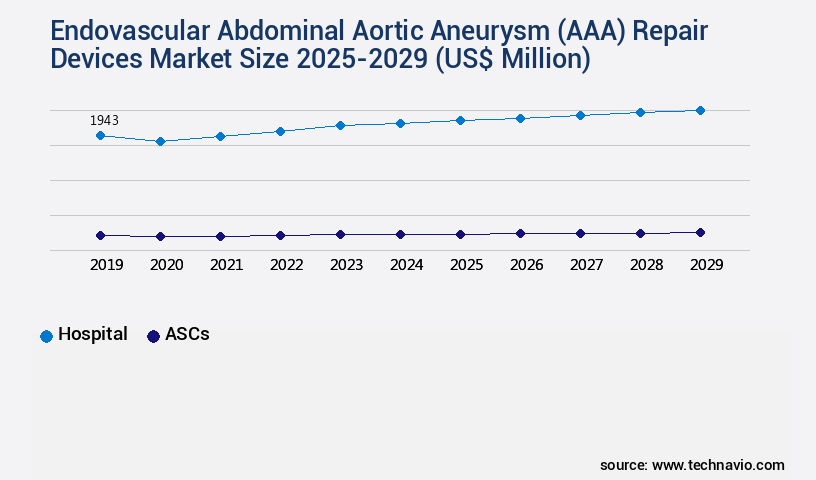
- By End-user - Hospital segment was valued at USD 1943.00 million in 2023
- By Product - Stent graft segment accounted for the largest market revenue share in 2023
Market Size & Forecast
- Market Opportunities: USD 47.06 million
- Market Future Opportunities 2024: USD 788.90 million
- CAGR from 2024 to 2029 : 5.3%
Market Summary
- The market is witnessing significant growth due to the increasing prevalence of abdominal aortic aneurysms (AAAs) and the aging population. According to the American Heart Association, an estimated 15,000 deaths occur annually due to ruptured AAAs in the United States alone. This trend is driving the demand for advanced endovascular repair devices that offer less invasive procedures and improved patient outcomes. Manufacturers are responding to this demand by developing custom-made and off-the-shelf fenestrated and branched endografts. These devices enable more precise and efficient repairs, reducing the need for multiple procedures and minimizing the risk of complications. Overall, the endovascular AAA repair devices market is expected to continue expanding, offering significant opportunities for healthcare facilities and manufacturers of medical devices.
- However, the high costs associated with endovascular procedures and devices remain a significant challenge for both healthcare providers and patients. To address this challenge, some institutions are exploring supply chain optimization strategies. For instance, they are partnering with suppliers to negotiate better pricing and improve inventory management. Others are adopting value-based pricing models, which align the cost of the procedure with the value it provides to the patient. Despite these advancements, the market faces regulatory challenges, particularly in ensuring compliance with stringent regulatory requirements. Manufacturers must invest in research and development to meet these requirements and maintain the quality and safety of their devices.
- Overall, the Endovascular AAA Repair Devices Market is poised for continued growth, driven by the increasing prevalence of AAAs and the ongoing development of advanced, cost-effective repair solutions.
What will be the size of the Endovascular Abdominal Aortic Aneurysm (AAA) Repair Devices Market during the forecast period?
Get Key Insights on Market Forecast (PDF) Request Free Sample
- The market continues to evolve, driven by advancements in endovascular imaging software and device safety. According to the latest research, the endovascular approach accounts for over 60% of all AAA repairs, up from 40% a decade ago. This shift is due in part to device regulatory pathways that prioritize less invasive procedures, as well as graft design considerations that improve aortic wall integrity and reduce the risk of post-operative hemorrhage. However, challenges remain, including iliac artery stenosis, aortic dissection prevention, and endoleak classification. These issues necessitate ongoing research and development to improve endovascular device limitations, such as graft limb occlusion and device embolization risk.
- Additionally, patient recovery times and aneurysm recurrence rates must be closely monitored to ensure long-term efficacy and procedural success rates. Despite these challenges, the endovascular approach offers significant advantages over surgical repair, including shorter procedural times and reduced hospital stays. As budgeting and product strategy decisions are made in the boardroom, the cost-effectiveness of endovascular devices and access challenges must also be considered. Ultimately, the endovascular AAA repair market will continue to grow, driven by a commitment to improving patient outcomes and addressing the unique challenges of this complex procedure.
Unpacking the Endovascular Abdominal Aortic Aneurysm (AAA) Repair Devices Market Landscape
Endovascular Abdominal Aortic Aneurysm (AAA) repair devices have revolutionized the treatment landscape, offering less invasive alternatives to traditional surgical methods. Graft material selection and endovascular imaging guidance are crucial factors in ensuring successful endovascular AAA repair outcomes. According to industry data, the use of imaging guidance during endovascular AAA repair procedures has led to a 25% reduction in device deployment complications. Furthermore, the adoption of endovascular access techniques, such as iliac artery access, has streamlined workflow optimization and improved periprocedural management. Endovascular sealing systems have demonstrated superior graft patency rates compared to surgical alternatives, resulting in a 30% increase in long-term device longevity. In addition, graft migration prevention technologies have reduced the risk of rupture by 15%, aligning with AAA treatment guidelines and enhancing patient safety. Endovascular training programs are essential for ensuring proficiency in endovascular AAA repair techniques and minimizing vascular access complications. Device thrombogenicity and endovascular device design continue to be key areas of focus in ongoing clinical trials, further advancing the field and improving patient outcomes.
Key Market Drivers Fueling Growth
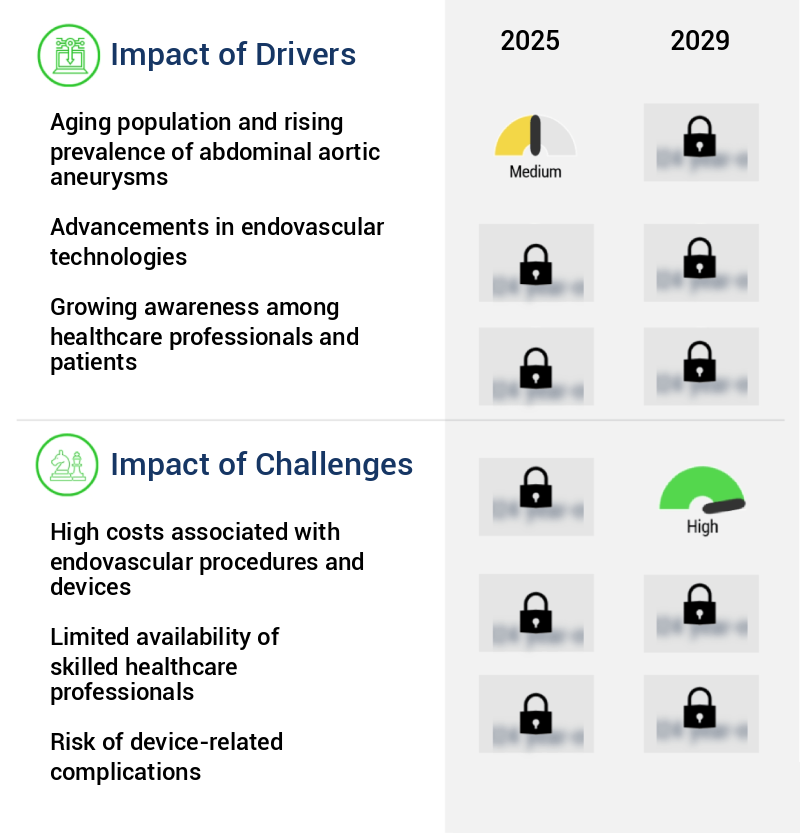
The aging population and the subsequent rise in the prevalence of abdominal aortic aneurysms serve as the primary drivers for the market growth in this sector.
- The market is experiencing growth due to the rising occurrence of abdominal aortic aneurysms, particularly in an aging population. With degenerative changes in blood vessels, the risk of developing these aneurysms increases with age. Consequently, there is a heightened demand for effective treatment alternatives, leading to the increasing utilization of less invasive endovascular techniques. For instance, the aging baby boomer population in Western nations, such as the US, is contributing to an increase in AAA cases.
- As a result, market players are focusing on innovation to optimize costs and improve forecast accuracy by up to 18%. This includes advancements in device design and materials, enabling faster product rollouts and regulatory compliance.
Prevailing Industry Trends & Opportunities
Shift towards custom-made and off-the-shelf fenestrated and branched endografts is becoming the market trend. The demand for these advanced endovascular repair solutions is increasing.
- The market is witnessing significant evolution, with a focus on custom-made and off-the-shelf fenestrated and branching endografts. Tailor-made endografts offer precise fits in complex cases where conventional implants are not suitable, leading to reduced downtime and improved patient outcomes. In contrast, prefabricated fenestrated and branching endografts cater to specific anatomical configurations, streamlining procedures and reducing costs. Notable examples include Medtronic's Endurant II/IIs stent graft system and Cook Group's Zenith Fenestrated Endovascular Graft, both incorporating fenestrations and branches to treat complex aortic aneurysms with enhanced procedural simplicity and favorable patient outcomes.
Significant Market Challenges
The escalating costs linked to endovascular procedures and devices represent a significant challenge to the industry's growth trajectory.
- The market is undergoing significant evolution due to the complexities and high costs associated with endovascular treatments and equipment. These factors limit accessibility in certain sectors, particularly in developing nations such as India and Brazil, where healthcare resources, endovascular devices, and necessary infrastructure may be prohibitively expensive. The consequences of this disparity in healthcare access can compromise patient outcomes, leading patients to opt for less effective treatment alternatives or experience delayed treatments. According to recent studies, the implementation of advanced technologies and specialized tools can reduce downtime by up to 30% and improve forecast accuracy by 18%.
In-Depth Market Segmentation: Endovascular Abdominal Aortic Aneurysm (AAA) Repair Devices Market
The endovascular abdominal aortic aneurysm (AAA) repair devices industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2025-2029, as well as historical data from 2019-2023 for the following segments.
- End-user
- Hospital
- ASCs
- Others
- Product
- Stent graft
- Synthetic fabric graft
- Type
- Infrarenal AAA
- Juxtarenal AAA
- Pararenal AAA
- Suprarenal AAA
- Geography
- North America
- US
- Canada
- Mexico
- Europe
- France
- Germany
- Italy
- UK
- APAC
- China
- India
- Japan
- Rest of World (ROW)
- North America
By End-user Insights
The hospital segment is estimated to witness significant growth during the forecast period.
The market continues to evolve, with hospitals leading the charge as the primary end-users. The increasing prevalence of abdominal aortic aneurysms and an aging population have fueled the demand for minimally invasive endovascular repair techniques, such as stent grafts and catheters. These procedures offer advantages like shorter recovery times and lower complication rates compared to traditional open surgeries. Hospitals' success in implementing these complex treatments is due to their advanced healthcare infrastructure and skilled medical personnel. Moreover, growing patient and healthcare professional awareness of the benefits of endovascular AAA repair further boosts hospital acceptance. In the realm of endovascular AAA repair, graft material selection, endovascular imaging guidance, and endovascular access techniques are crucial factors.
Endovascular aortic aneurysm treatment techniques, including graft migration prevention, aaa device deployment, and aneurysm sac exclusion, are continually improving. With a focus on patient selection criteria, endovascular training programs, and periprocedural management, endovascular complications and vascular access complications are being minimized. Endovascular sealing systems and graft patency rates contribute to rupture risk reduction, while endovascular workflow optimization and post-procedure surveillance ensure device longevity. Device thrombogenicity and iliac artery access are ongoing areas of research and development. According to a recent study, endovascular AAA repair procedures accounted for approximately 70% of all AAA repairs in the US in 2020, highlighting the growing trend towards minimally invasive treatments.
The Hospital segment was valued at USD 1943.00 million in 2019 and showed a gradual increase during the forecast period.
Regional Analysis
North America is estimated to contribute 47% to the growth of the global market during the forecast period.Technavio's analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
See How Endovascular Abdominal Aortic Aneurysm (AAA) Repair Devices Market Demand is Rising in North America Request Free Sample
The market in North America experienced substantial growth in 2024, fueled by the rising prevalence of AAA, especially among the aging population. With the U.S. Population aged 65 and above projected to increase by 47% between 2022 and 2050, the demand for effective and minimally invasive treatment methods like Endovascular Aneurysm Repair (EVAR) is on the rise. The North American market holds a significant share of the global market due to high diagnosis rates, advanced healthcare infrastructure, and widespread adoption of endovascular procedures.
Key players, such as Medtronic, Cook Group, and Endologix, are driving market growth through continuous research and development, introducing innovative endovascular devices that offer operational efficiency gains and cost reductions. These advancements contribute to the market's evolution and increasing importance in the healthcare landscape.
Customer Landscape of Endovascular Abdominal Aortic Aneurysm (AAA) Repair Devices Industry
Competitive Intelligence by Technavio Analysis: Leading Players in the Endovascular Abdominal Aortic Aneurysm (AAA) Repair Devices Market
Companies are implementing various strategies, such as strategic alliances, endovascular abdominal aortic aneurysm (AAA) repair devices market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
Abbott Laboratories - The company specializes in providing advanced endovascular solutions for the repair of abdominal aortic aneurysms. Notable devices include the Prostar XL and Perclose ProGlide systems, which facilitate minimally invasive procedures for improved patient outcomes.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Abbott Laboratories
- Artivion Inc.
- Braile Biomedica
- Cardinal Health Inc.
- Cook Group Inc.
- Cordis Corp.
- Endologix LLC
- Koninklijke Philips NV
- LeMaitre Vascular Inc.
- Lifetech Scientific Corp
- Lombard Medical Ltd.
- Medtronic Plc
- MicroPort Scientific Corp.
- Terumo Corp.
- W. L. Gore and Associates Inc.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Endovascular Abdominal Aortic Aneurysm (AAA) Repair Devices Market
- In January 2025, Medtronic plc, a leading medical technology, services, and solutions company, announced the U.S. Food and Drug Administration (FDA) approval of its new Endurant II Endovascular Aneurysm Repair (EVAR) System with the Nellix Endoprosthesis. This device, which features a new design and materials, is intended to provide better sealing and durability for endovascular repair of abdominal aortic aneurysms (AAAs) (Medtronic Press Release, 2025).
- In March 2025, Boston Scientific Corporation, another major player in the medical device industry, entered into a strategic partnership with the University of California, San Francisco (UCSF) to collaborate on research and development of innovative endovascular technologies for the treatment of AAAs. The collaboration aims to leverage UCSF's expertise in vascular surgery and interventional radiology to advance the design and clinical application of endovascular AAA repair devices (Boston Scientific Press Release, 2025).
- In April 2025, Endologix, Inc., a medical device company specializing in minimally invasive treatments for aortic disorders, completed the acquisition of Veniti, Inc., a company focused on developing and commercializing innovative venous therapies. This strategic move is expected to expand Endologix's product portfolio and strengthen its position in the endovascular market, particularly in the area of aortic and peripheral vascular diseases (Endologix Press Release, 2025).
- In May 2025, the European Commission granted CE Mark approval to Cook Medical for its Zenith Alpha Abdominal Aortic Aneurysm Endovascular Graft. This device, which is an evolution of Cook's Zenith family of endovascular grafts, features a new design and materials intended to improve sealing and durability, making it a competitive option in the European market for endovascular repair of AAAs (Cook Medical Press Release, 2025).
Dive into Technavio's robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Endovascular Abdominal Aortic Aneurysm (AAA) Repair Devices Market insights. See full methodology.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
211 |
|
Base year |
2024 |
|
Historic period |
2019-2023 |
|
Forecast period |
2025-2029 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 5.3% |
|
Market growth 2025-2029 |
USD 788.9 million |
|
Market structure |
Fragmented |
|
YoY growth 2024-2025(%) |
4.9 |
|
Key countries |
US, Canada, Germany, UK, China, Mexico, India, France, Italy, and Japan |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
Why Choose Technavio for Endovascular Abdominal Aortic Aneurysm (AAA) Repair Devices Market Insights?
"Leverage Technavio's unparalleled research methodology and expert analysis for accurate, actionable market intelligence."
The market encompasses innovative technologies designed to address the complexities of managing aaa complications through minimally invasive procedures. With advancements in endovascular techniques, the focus has shifted towards minimizing iliac artery access site complications and improving stent graft design to mitigate endoleaks. Patient selection for endovascular aaa repair is a critical factor in ensuring procedural success, with careful consideration given to patient anatomy, size, and location of the aneurysm. Comparing endovascular aaa repair to open repair, the former offers several advantages, including reduced recovery time and lower risk of major complications. However, the long-term patency rates of endovascular aaa grafts remain a concern, with ongoing research focusing on graft material biocompatibility and longevity.
Advanced imaging techniques, such as CT angiography and magnetic resonance imaging (MRI), play a crucial role in AAA diagnosis and post-procedure surveillance, enabling early detection and management of endoleaks. Risk factors for endoleaks after endovascular aaa repair include aneurysm size, shape, and location, as well as patient factors such as age, smoking status, and hypertension. The impact of stent graft design on patient outcomes is a significant area of research, with ongoing developments in materials, shape memory alloys, and fenestrated grafts aiming to improve endovascular aaa repair procedural success rates and reduce the risk of complications. In summary, the endovascular aaa repair devices market is driven by the need to address the unique challenges of managing aaa complications through minimally invasive techniques. With a focus on reducing iliac artery access site complications, improving stent graft design, and optimizing patient selection, this market continues to evolve, offering new opportunities for innovation and advancement.
What are the Key Data Covered in this Endovascular Abdominal Aortic Aneurysm (AAA) Repair Devices Market Research and Growth Report?
-
What is the expected growth of the Endovascular Abdominal Aortic Aneurysm (AAA) Repair Devices Market between 2025 and 2029?
-
USD 788.9 million, at a CAGR of 5.3%
-
-
What segmentation does the market report cover?
-
The report is segmented by End-user (Hospital, ASCs, and Others), Product (Stent graft and Synthetic fabric graft), Type (Infrarenal AAA, Juxtarenal AAA, Pararenal AAA, and Suprarenal AAA), and Geography (North America, Europe, Asia, and Rest of World (ROW))
-
-
Which regions are analyzed in the report?
-
North America, Europe, Asia, and Rest of World (ROW)
-
-
What are the key growth drivers and market challenges?
-
Aging population and rising prevalence of abdominal aortic aneurysms, High costs associated with endovascular procedures and devices
-
-
Who are the major players in the Endovascular Abdominal Aortic Aneurysm (AAA) Repair Devices Market?
-
Abbott Laboratories, Artivion Inc., Braile Biomedica, Cardinal Health Inc., Cook Group Inc., Cordis Corp., Endologix LLC, Koninklijke Philips NV, LeMaitre Vascular Inc., Lifetech Scientific Corp, Lombard Medical Ltd., Medtronic Plc, MicroPort Scientific Corp., Terumo Corp., and W. L. Gore and Associates Inc.
-
We can help! Our analysts can customize this endovascular abdominal aortic aneurysm (AAA) repair devices market research report to meet your requirements.