Exosome Therapeutics Market Size 2026-2030
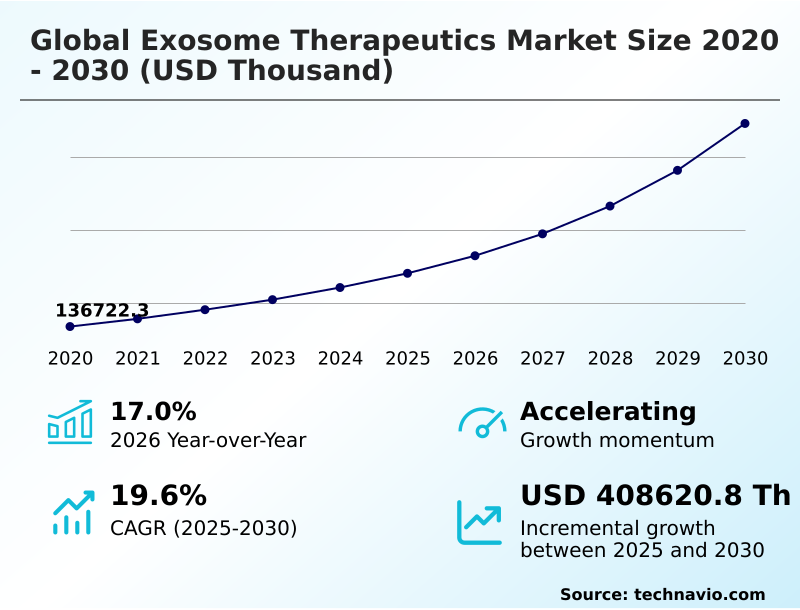
The exosome therapeutics market size is valued to increase by USD 408.62 million, at a CAGR of 19.6% from 2025 to 2030. Increasingly sophisticated and scalable manufacturing technologies will drive the exosome therapeutics market.
Major Market Trends & Insights
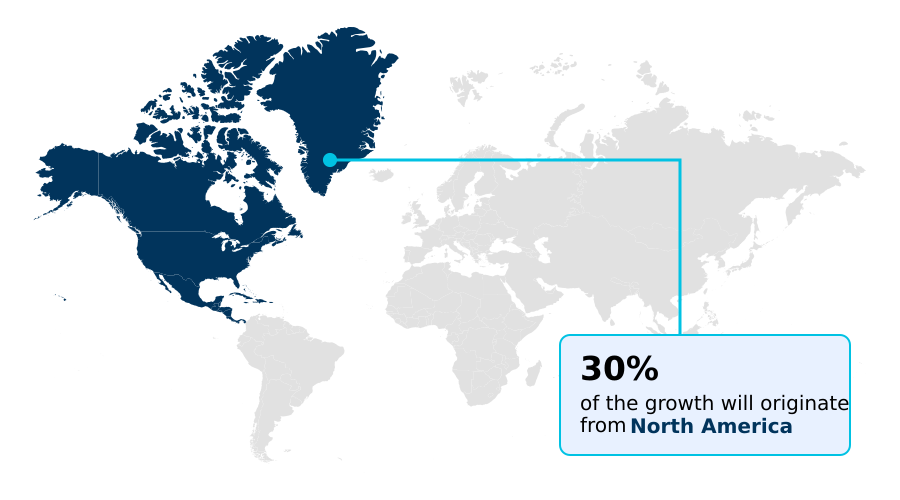
- North America dominated the market and accounted for a 29.7% growth during the forecast period.
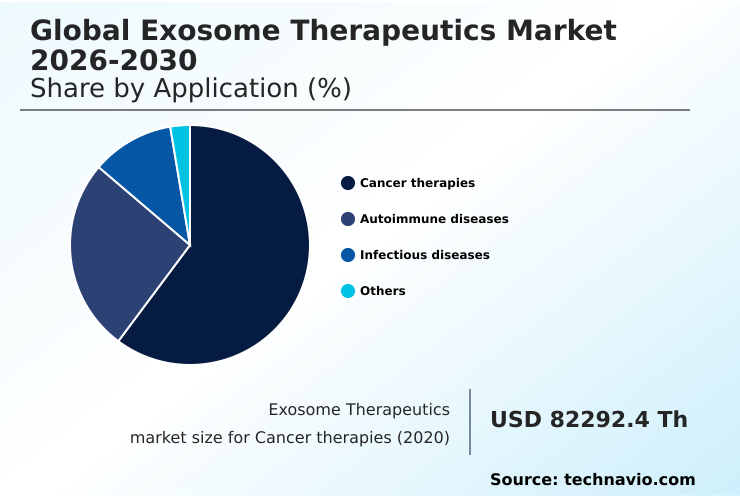
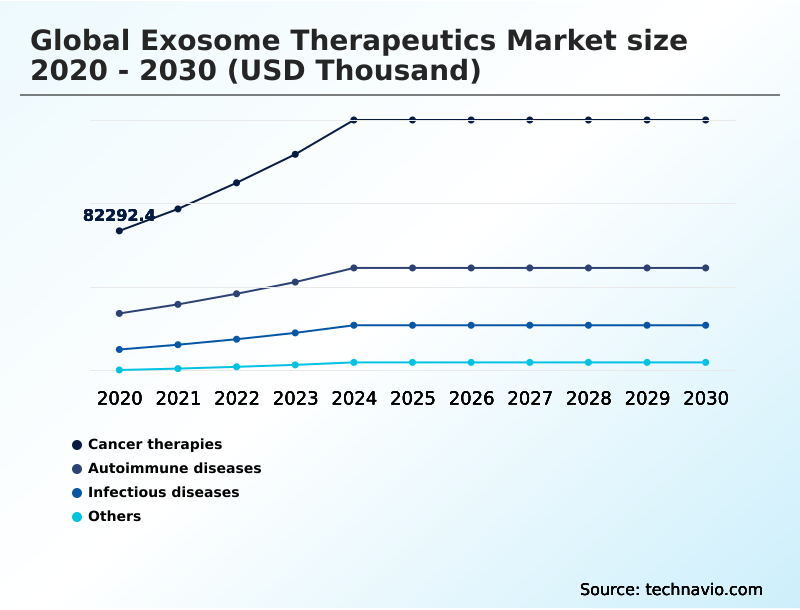
- By Application - Cancer therapies segment was valued at USD 144.97 million in 2024
- By Distribution Channel - Hospital pharmacy segment accounted for the largest market revenue share in 2024
Market Size & Forecast
- Market Opportunities:
- Market Future Opportunities: USD 408.62 million
- CAGR from 2025 to 2030 : 19.6%
Market Summary
- The exosome therapeutics market is undergoing a significant transformation, moving beyond academic research toward clinical and commercial viability. This shift is centered on harnessing the natural intercellular communication capabilities of these vesicles for therapeutic purposes. Key progress is seen in the clinical pipeline expansion and the development of exosome-based vaccines.
- For instance, a biotech firm navigating the path to commercialization must decide between building in-house gmp manufacturing of exosomes capabilities or partnering with an exosome contract manufacturing organization. This decision directly impacts capital expenditure and speed to market, with outsourced partnerships often reducing time-to-clinic by up to 25%.
- While the promise of cell-free therapy is immense, particularly for regenerative medicine and rna-based therapies, the industry must address persistent challenges. These include establishing robust exosome isolation techniques and meeting the stringent requirements for an investigational new drug application and eventual biologics license application.
- The evolution from research-grade to clinical-grade production is a critical hurdle, defining the pace of market adoption and the successful translation of nanovesicle drug delivery platforms into approved treatments for patients.
What will be the Size of the Exosome Therapeutics Market during the forecast period?
Get Key Insights on Market Forecast (PDF) Request Free Sample
How is the Exosome Therapeutics Market Segmented?
The exosome therapeutics industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD thousand" for the period 2026-2030, as well as historical data from 2020-2024 for the following segments.
- Application
- Cancer therapies
- Autoimmune diseases
- Infectious diseases
- Others
- Distribution channel
- Hospital pharmacy
- Retail pharmacy
- Online pharmacy
- Type
- Allogeneic
- Autologous
- Geography
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Asia
- Rest of World (ROW)
- North America
By Application Insights
The cancer therapies segment is estimated to witness significant growth during the forecast period.
The cancer therapies segment is a central pillar of the exosome therapeutics market, where these vesicles are critical in both disease progression and as a treatment modality.
The focus on targeted drug delivery is paramount, utilizing exosome engineering to create vehicles for therapeutic payload delivery to tumor sites, which enhances efficacy. Innovations in allogeneic vs autologous exosomes approaches are defining personalized medicine strategies.
Leveraging their immunomodulatory properties for oncology applications is a key research area, particularly for exosome-based treatment for cancer.
Advancements in exosome purification methods are improving yields by over 25% compared to legacy techniques, enabling more robust biomarker discovery and development of extracellular vesicle therapeutics for this application, including for exosomes in neurodegenerative diseases.
The Cancer therapies segment was valued at USD 144.97 million in 2024 and showed a gradual increase during the forecast period.
Regional Analysis
North America is estimated to contribute 29.7% to the growth of the global market during the forecast period.Technavio’s analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
See How Exosome Therapeutics Market Demand is Rising in North America Request Free Sample
The geographic landscape of the exosome therapeutics market is led by North America, which is projected to account for nearly 30% of the incremental growth, closely followed by Europe at just over 26%.
This dominance is driven by a robust R&D ecosystem and significant venture capital funding. In Europe, collaborative research is advancing the use of mesenchymal stem cell exosomes and anti-inflammatory exosomes.
Asia is the fastest-growing region, with countries like South Korea and Japan making significant strides in both regulatory approvals and exosome cGMP guidelines for exosome cargo engineering.
This regional growth is fueled by government support for biotechnology and an increasing number of local firms developing proprietary platforms for exosome-based diagnostics and therapies.
The disparity in clinical trial for exosomes activity, with North America leading, underscores the different stages of market maturity across regions.
Market Dynamics
Our researchers analyzed the data with 2025 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
- The commercialization of the exosome therapeutics market hinges on resolving several complex technical and regulatory issues. Significant advancements in exosome manufacturing technology are critical for scaling production from laboratory to clinical-grade quantities, a transition that remains a primary bottleneck for many developers.
- The therapeutic promise is immense, particularly for indications like exosome therapy for acute ischemic stroke, where the ability of these vesicles to cross the blood-brain barrier offers a unique advantage over traditional biologics. However, demonstrating the clinical efficacy of msc-derived exosomes in large-scale human trials is a crucial next step that requires substantial investment and time.
- Adding to the complexity are the regulatory challenges for exosome therapeutics, as agencies worldwide work to establish clear guidelines for this novel class of therapy. This ambiguity can delay development timelines, with some firms reporting that navigating differing international requirements extends their pre-market phase by up to 18 months compared to more established drug classes.
- Furthermore, the successful exosome-based delivery of sirna and mrna represents a major opportunity, positioning exosomes as a key platform technology in the future of genetic medicine and personalized treatments. Addressing these interconnected challenges is essential for unlocking the full potential of this innovative field.
What are the key market drivers leading to the rise in the adoption of Exosome Therapeutics Industry?
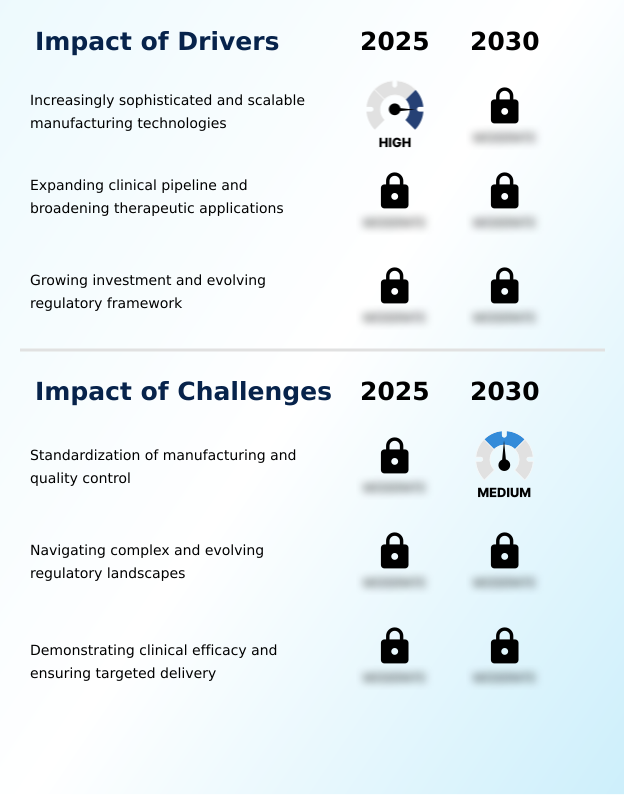
- Increasingly sophisticated and scalable manufacturing technologies are a key driver of market growth.
- Market growth is significantly driven by advancements in manufacturing, where new exosome purification methods are improving yields by up to 50%.
- This addresses historical bottlenecks in gmp manufacturing of exosomes and enables the development of commercially viable advanced therapy medicinal products. A diversifying clinical pipeline expansion, moving into indications such as cardioprotective exosomes and neurodegenerative diseases, is attracting substantial investment.
- Early-stage funding rounds are reportedly 30% larger year-over-year. Concurrently, a clearer regulatory pathways for exosomes framework is reducing uncertainty for developers.
- This is crucial for advancing products through the investigational new drug application and biologics license application stages, fostering a stable environment for innovation in cell-free therapy and allogeneic exosome therapy.
What are the market trends shaping the Exosome Therapeutics Industry?
- The expansion of exosomes as sophisticated drug delivery systems represents a significant upcoming market trend. This is driven by their inherent ability to transport therapeutic payloads to targeted cells.
- A primary trend shaping the exosome therapeutics market is the engineering of these vesicles into sophisticated exosome drug delivery systems. This approach is enhancing therapeutic payload delivery, with some studies showing a reduction in off-target effects by over 40% compared to conventional methods.
- The development of artificial and hybrid exosomes is addressing limitations in exosome manufacturing scale-up, allowing for greater consistency and control. This innovation is critical for exosome therapeutic applications in complex areas like oncology and neurodegeneration. Furthermore, rising partnerships are accelerating development timelines by an average of 25%, pooling expertise in areas like exosome cargo engineering and regulatory navigation.
- This collaborative trend is expanding the utility of autologous exosome therapy and exosomes for autoimmune disorders.
What challenges does the Exosome Therapeutics Industry face during its growth?
- The lack of standardization in manufacturing and quality control presents a key challenge to industry growth.
- A primary challenge for the market is establishing standardized exosome quality control standards to manage product heterogeneity. Batch-to-batch variability, often exceeding 20%, complicates the path to regulatory approval and hinders the development of reliable nanovesicle drug delivery platforms.
- Navigating complex and fragmented exosome cGMP guidelines across different regions can extend development timelines by up to 18 months, posing a significant risk for developers of mesenchymal stem cell exosomes. Furthermore, demonstrating clinical efficacy and understanding exosome stability and storage, exosome pharmacokinetics, and biodistribution remain critical hurdles.
- The industry must move beyond preclinical success to deliver consistent outcomes in human exosome therapy clinical trials, which is essential for building confidence in this novel therapeutic modality.
Exclusive Technavio Analysis on Customer Landscape
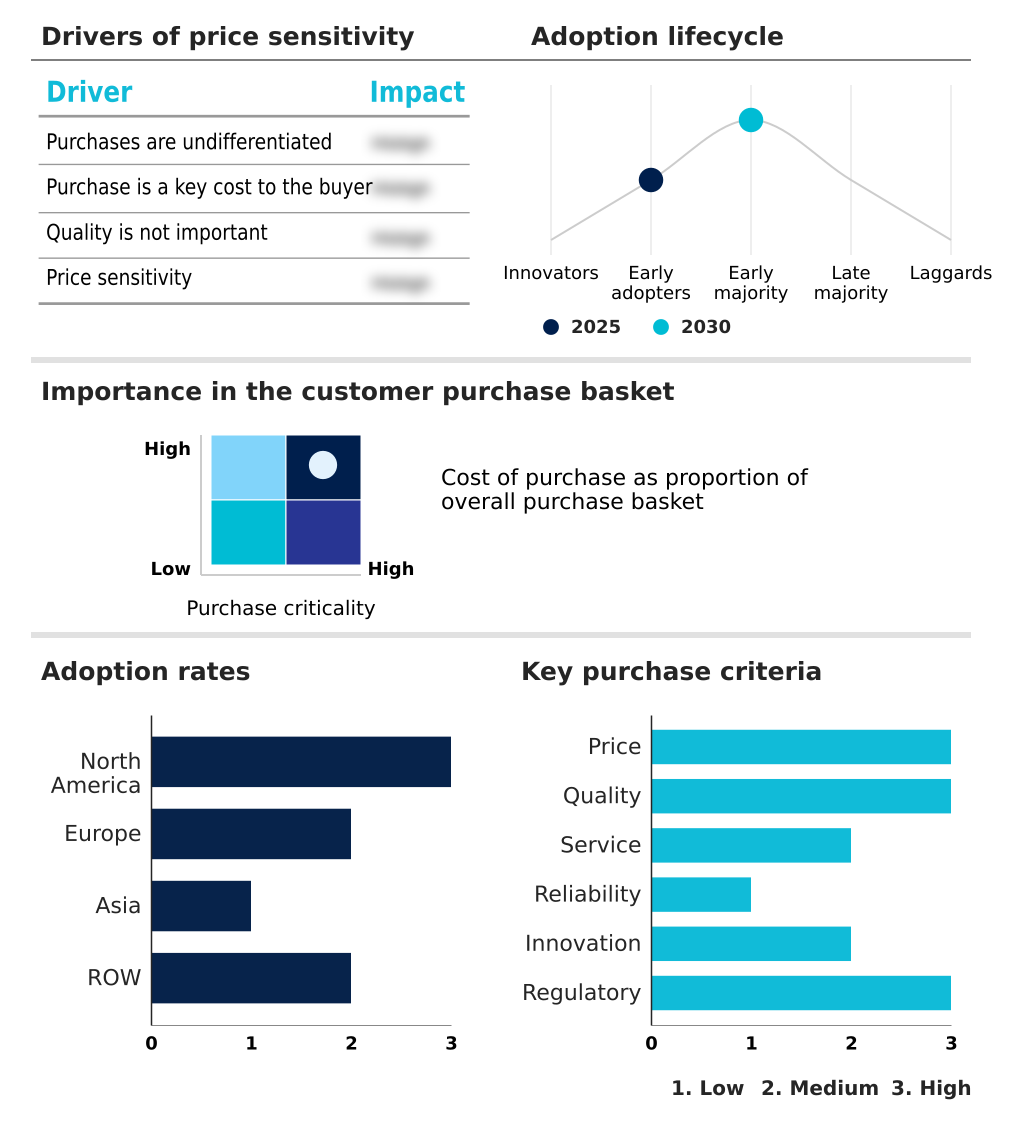
The exosome therapeutics market forecasting report includes the adoption lifecycle of the market, covering from the innovator’s stage to the laggard’s stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the exosome therapeutics market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape of Exosome Therapeutics Industry
Competitive Landscape
Companies are implementing various strategies, such as strategic alliances, exosome therapeutics market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
Aegle Therapeutics - Focus is on developing extracellular vesicle-based therapies for dermatological conditions, utilizing regenerative medicine to modulate tissue repair and address inflammatory responses.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Aegle Therapeutics
- AMS Biotechnology Europe Ltd.
- Aruna Bio Inc.
- Capricor Therapeutics Inc.
- CIloa SAS
- Creative Biolabs
- Cytiva
- Direct Biologics LLC
- Evotec SE
- Evox Therapeutics Ltd.
- ExoCoBio Inc.
- ILIAS Biologics Inc.
- Lonza Group Ltd.
- NanoFCM Co. Ltd
- ReNeuron Group plc
- Rion Inc.
- Thermo Fisher Scientific Inc.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Exosome therapeutics market
- In August 2024, Capricor Therapeutics announced a successful pre-Biologics License Application meeting with the US FDA for its candidate CAP-1002, aimed at treating Duchenne muscular dystrophy.
- In January 2025, NurExone Biologic announced the acquisition of a master cell bank from a US-based manufacturer to secure and scale its supply chain for exosome-based therapies.
- In April 2025, RoosterBio Inc. and Thermo Fisher Scientific announced a collaboration to accelerate the manufacturing of cell and exosome therapies, addressing scalability and cost challenges.
- In May 2025, South Korea's Ministry of Food and Drug Safety granted approval to S and E bio Co. Ltd. to commence a Phase 1b clinical trial for an exosome-based stroke treatment.
Dive into Technavio’s robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Exosome Therapeutics Market insights. See full methodology.
| Market Scope | |
|---|---|
| Page number | 284 |
| Base year | 2025 |
| Historic period | 2020-2024 |
| Forecast period | 2026-2030 |
| Growth momentum & CAGR | Accelerate at a CAGR of 19.6% |
| Market growth 2026-2030 | USD 408620.8 thousand |
| Market structure | Fragmented |
| YoY growth 2025-2026(%) | 17.0% |
| Key countries | US, Canada, Mexico, Germany, UK, France, Italy, Spain, The Netherlands, China, Japan, India, South Korea, Thailand, Indonesia, Brazil, Saudi Arabia, South Africa, UAE, Israel, Argentina, Colombia and Turkey |
| Competitive landscape | Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
Research Analyst Overview
- The exosome therapeutics market is advancing rapidly, driven by innovation in exosome isolation techniques such as tangential flow filtration and size exclusion chromatography. These methods are pivotal for ensuring the purity and consistency required for gmp manufacturing of exosomes.
- A key boardroom-level decision is whether to invest in in-house manufacturing or leverage specialized contract development and manufacturing organizations, a choice that significantly impacts capital allocation and speed to market. This move towards professionalized production is enabling a notable clinical pipeline expansion, with a focus on allogeneic exosome therapy as a scalable, off-the-shelf solution.
- Advancements in exosome characterization and exosome drug loading are allowing for more precise targeted drug delivery, including for rna-based therapies. This progress is critical for demonstrating efficacy in exosome-based treatment for cancer and realizing the potential of the neural exosome platform for blood-brain barrier penetration.
- The industry is moving from autologous exosome therapy toward more commercially viable models, underpinned by robust biomarker discovery and the development of exosome-based vaccines and other forms of cell-free therapy for regenerative medicine and extracellular vesicle therapeutics.
What are the Key Data Covered in this Exosome Therapeutics Market Research and Growth Report?
-
What is the expected growth of the Exosome Therapeutics Market between 2026 and 2030?
-
USD 408.62 million, at a CAGR of 19.6%
-
-
What segmentation does the market report cover?
-
The report is segmented by Application (Cancer therapies, Autoimmune diseases, Infectious diseases, and Others), Distribution Channel (Hospital pharmacy, Retail pharmacy, and Online pharmacy), Type (Allogeneic, and Autologous) and Geography (North America, Europe, Asia, Rest of World (ROW))
-
-
Which regions are analyzed in the report?
-
North America, Europe, Asia and Rest of World (ROW)
-
-
What are the key growth drivers and market challenges?
-
Increasingly sophisticated and scalable manufacturing technologies, Standardization of manufacturing and quality control
-
-
Who are the major players in the Exosome Therapeutics Market?
-
Aegle Therapeutics, AMS Biotechnology Europe Ltd., Aruna Bio Inc., Capricor Therapeutics Inc., CIloa SAS, Creative Biolabs, Cytiva, Direct Biologics LLC, Evotec SE, Evox Therapeutics Ltd., ExoCoBio Inc., ILIAS Biologics Inc., Lonza Group Ltd., NanoFCM Co. Ltd, ReNeuron Group plc, Rion Inc. and Thermo Fisher Scientific Inc.
-
Market Research Insights
- The exosome therapeutics market is shaped by dynamic interactions between technological innovation and regulatory evolution. The development of advanced therapy medicinal products is accelerating, with exosome therapeutic applications expanding into new disease areas. Collaborations are proving crucial, with strategic partnerships reducing early-stage R&D timelines by an estimated 20%.
- The industry is focused on exosome manufacturing scale-up, with new methods yielding purity levels over 95%, a significant improvement that supports exosome stability and storage. As exosome therapy clinical trials progress, there is a clear push towards establishing exosome quality control standards to ensure product consistency and safety.
- Understanding exosome pharmacokinetics and exosome biodistribution studies is essential for validating efficacy and gaining regulatory approval. The focus on dermatological exosome applications and cardioprotective exosomes highlights the diversification of the clinical pipeline beyond oncology.
We can help! Our analysts can customize this exosome therapeutics market research report to meet your requirements.