Guidewires Market Size 2026-2030
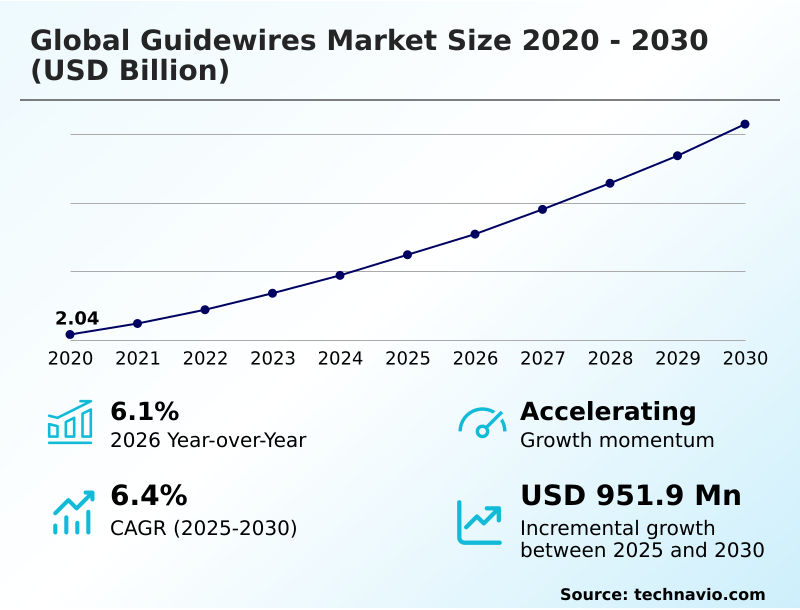
The guidewires market size is valued to increase by USD 951.9 million, at a CAGR of 6.4% from 2025 to 2030. Escalating global burden of chronic and age related diseases will drive the guidewires market.
Major Market Trends & Insights
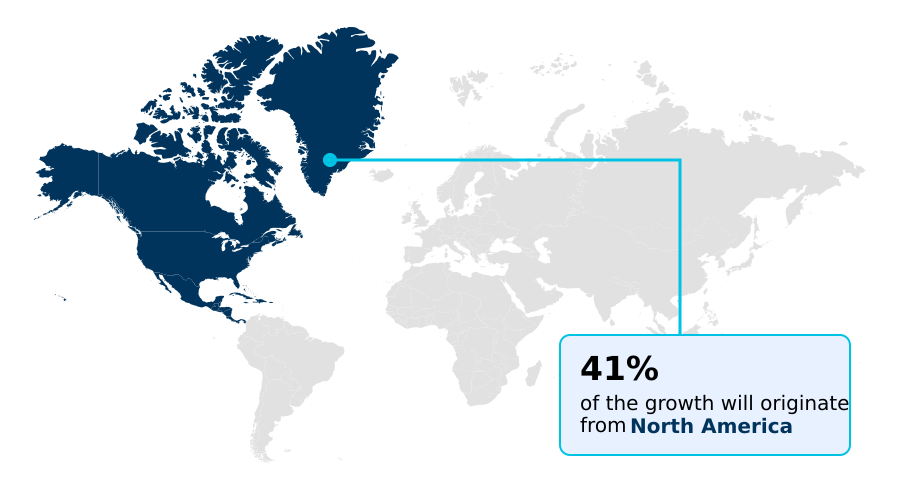
- North America dominated the market and accounted for a 40.6% growth during the forecast period.
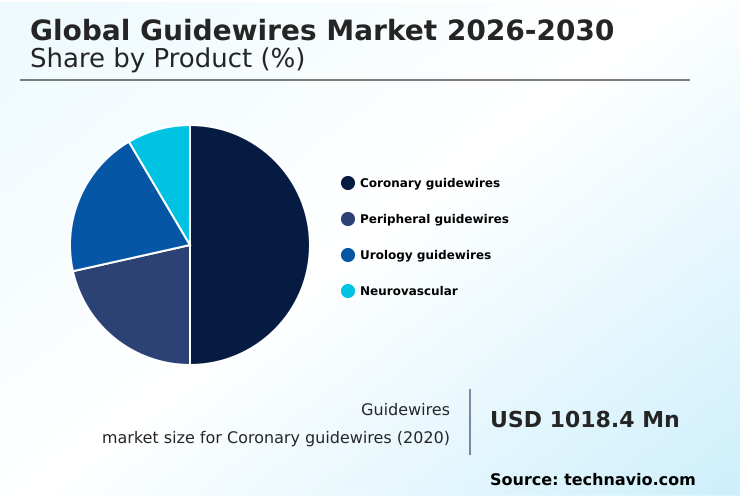
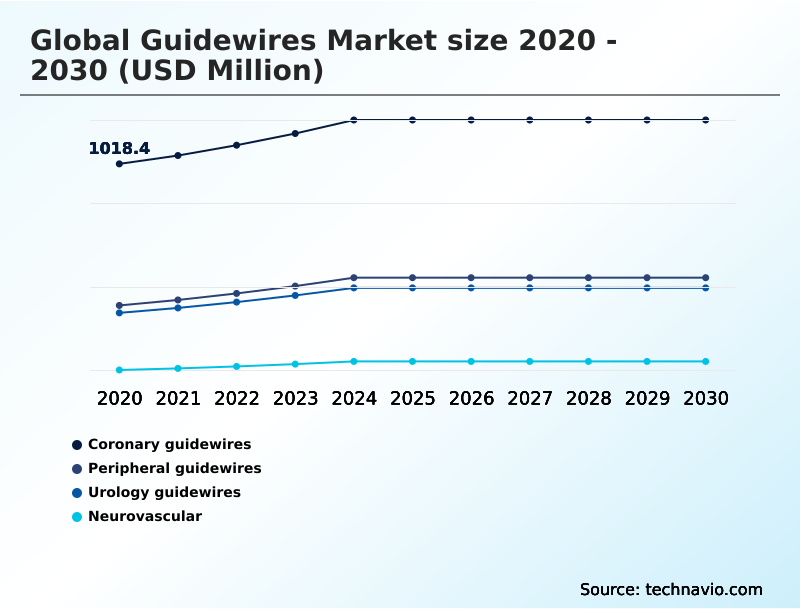
- By Product - Coronary guidewires segment was valued at USD 1.20 billion in 2024
- By End-user - Cardiac catheterization laboratories segment accounted for the largest market revenue share in 2024
Market Size & Forecast
- Market Opportunities: USD 1.53 billion
- Market Future Opportunities: USD 951.9 million
- CAGR from 2025 to 2030 : 6.4%
Market Summary
- The guidewires market is shaped by the imperative for safer and more effective minimally invasive procedures across numerous medical disciplines. Market expansion is fundamentally tied to the rising prevalence of chronic conditions and the broad clinical shift away from open surgery. This dynamic creates sustained demand for increasingly sophisticated devices that enable access to complex anatomies.
- Technological evolution, focusing on advanced materials and coatings, is a critical growth pillar, allowing clinicians to treat pathologies that were previously inaccessible. However, this innovation occurs within a landscape of rigorous regulatory oversight and significant cost containment pressures from healthcare systems.
- A key business challenge involves navigating this environment, for instance, by providing robust clinical evidence to justify the adoption of a premium-priced specialty wire. Success depends on aligning product development with the demands of both procedural complexity and the economic realities of modern healthcare, including the need for supply chain efficiency and effective inventory management.
What will be the Size of the Guidewires Market during the forecast period?
Get Key Insights on Market Forecast (PDF) Request Free Sample
How is the Guidewires Market Segmented?
The guidewires industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2026-2030, as well as historical data from 2020-2024 for the following segments.
- Product
- Coronary guidewires
- Peripheral guidewires
- Urology guidewires
- Neurovascular
- End-user
- Cardiac catheterization laboratories
- Hospitals
- Specialty clinics
- Ambulatory surgical centers
- Type
- Hydrophilic
- Uncoated
- Hydrophobic
- Geography
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Asia
- Rest of World (ROW)
- North America
By Product Insights
The coronary guidewires segment is estimated to witness significant growth during the forecast period.
The coronary guidewires segment is foundational, driven by the high volume of procedures in cardiac catheterization laboratory settings. These devices are crucial for percutaneous coronary interventions, requiring a delicate balance of performance characteristics.
The market is stratified into workhorse wires for routine cases and specialized models for complex anatomies. Innovation is increasingly centered on hybrid designs that improve procedural efficiency.
The selection process is influenced by physician preference and value analysis committee review, with advanced guidewires demonstrating the ability to reduce procedural times by over 15%.
This emphasis on clinical outcomes and cost-effectiveness shapes procurement within this mature market segment, where device performance is paramount for navigating tortuous coronary anatomy and ensuring successful treatment.
The Coronary guidewires segment was valued at USD 1.20 billion in 2024 and showed a gradual increase during the forecast period.
Regional Analysis
North America is estimated to contribute 40.6% to the growth of the global market during the forecast period.Technavio’s analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
See How Guidewires Market Demand is Rising in North America Request Free Sample
The geographic landscape is diverse, with North America representing the most mature market, driven by high healthcare expenditure and rapid technology adoption. It accounts for over 40% of incremental growth opportunities.
In contrast, Asia is the fastest-growing region, with its market expanding at a rate 1.3 times that of Europe, fueled by rising incomes and investments in healthcare infrastructure. However, centralized procurement policies in key Asian markets create significant price pressure.
Europe's market is shaped by stringent medical device regulation (MDR) and varying reimbursement models across countries, demanding regulatory harmonization.
The Rest of World (ROW) region presents a mosaic of opportunities, from high-spending markets in the Middle East to developing economies in South America, all gradually adopting global standards to ensure device safety and facilitate trade.
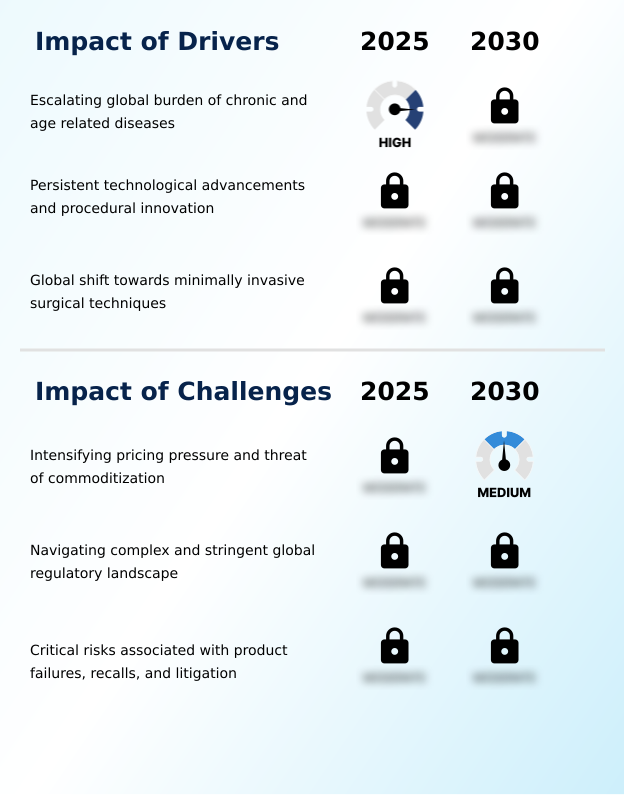
Market Dynamics
Our researchers analyzed the data with 2025 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
- Strategic decision-making in the guidewires market requires a deep understanding of nuanced procedural challenges and economic factors. The debate over hydrophilic vs hydrophobic guidewire coatings is central to clinical practice, with choices impacting performance in specific scenarios. For instance, guidewire selection for chronic total occlusions is a sub-specialty, demanding tools with exceptional crossing power.
- The technical properties of a device, such as nitinol core guidewire kink resistance and its performance in tortuous anatomy, are critical. A major concern is the coating delamination risk in guidewires, which necessitates robust quality control and biocompatibility testing for guidewire materials. For manufacturers, enhancing guidewire trackability and pushability is a constant R&D focus.
- The evolving guidewire role in transcatheter valve delivery and its use in pediatric interventional cardiology are expanding applications. Optimizing guidewire support for peripheral interventions and developing specific neurovascular guidewires for aneurysm coiling or urology guidewires for ureteroscopy procedures highlight the trend towards specialization. The impact of guidewire stiffness on performance must be balanced with safety to prevent guidewire-induced vessel trauma.
- For providers, the cost-effectiveness of specialty guidewires is a key consideration, and efficiently managing guidewire inventory in cath labs can reduce holding costs by over 20%. Navigating the regulatory approval for new guidewire designs, understanding guidewire compatibility with imaging catheters, and reducing friction in catheter delivery systems are all crucial.
- Ultimately, achieving superior steerability and torque response in guidewires is what enables complex, life-saving interventions.
What are the key market drivers leading to the rise in the adoption of Guidewires Industry?
- The escalating global burden of chronic and age-related diseases is a key driver fueling demand within the market.
- Market growth is fundamentally driven by the rising global prevalence of chronic diseases and the accelerating adoption of minimally invasive techniques. An aging population contributes to a higher incidence of complex vascular conditions, creating sustained demand for advanced interventional tools.
- The definitive shift from open surgery to catheter-based interventions offers compelling benefits, including reduced patient trauma and shorter hospital stays, which can lower the total episode of care cost by up to 30%.
- This paradigm shift makes guidewires essential as the foundational tool for nearly every endovascular, urological, and neurointerventional procedure.
- Technological innovation acts as a powerful catalyst, with advancements in materials and coatings expanding the range of treatable conditions and improving both the safety and procedural efficiency of interventions.
What are the market trends shaping the Guidewires Industry?
- A defining market trend is the proliferation of application-specific guidewires designed for complex interventions. This shift addresses the growing need for specialized tools to treat challenging clinical scenarios.
- Key market trends reflect a strategic shift toward specialization and integrated care delivery. The development of application-specific guidewires for complex interventions addresses the needs of challenging clinical scenarios, improving lesion crossing success in chronic total occlusions by up to 25%. This move away from one-size-fits-all tools allows for better performance in niche applications.
- Concurrently, manufacturers are adopting a procedural solution model, bundling guidewires with compatible devices to streamline procurement and enhance performance, which can reduce administrative overhead for a value analysis committee by 15%.
- A third transformative trend is the migration of appropriate cases to outpatient settings, a structural shift in the site of care driven by economic incentives and patient preference, further altering purchasing dynamics.
What challenges does the Guidewires Industry face during its growth?
- Intensifying pricing pressure and the pervasive threat of commoditization present a key challenge to industry growth.
- The market faces significant headwinds from intense pricing pressure and the threat of commoditization, driven by systemic cost containment efforts in global healthcare. The influence of large group purchasing organizations often results in price reductions of 10-15% on high-volume contracts. Simultaneously, navigating the complex and non-harmonized global regulatory landscape is a major operational challenge.
- The stringent requirements of frameworks like the EU's Medical Device Regulation have increased the documentation and clinical evidence burden for manufacturers by an estimated 40%. This environment makes it difficult to justify premium pricing without robust data on clinical and economic benefits.
- Furthermore, the inherent risks of product failures, potential recalls, and product liability litigation demand massive investments in quality management systems, adding further cost and complexity.
Exclusive Technavio Analysis on Customer Landscape
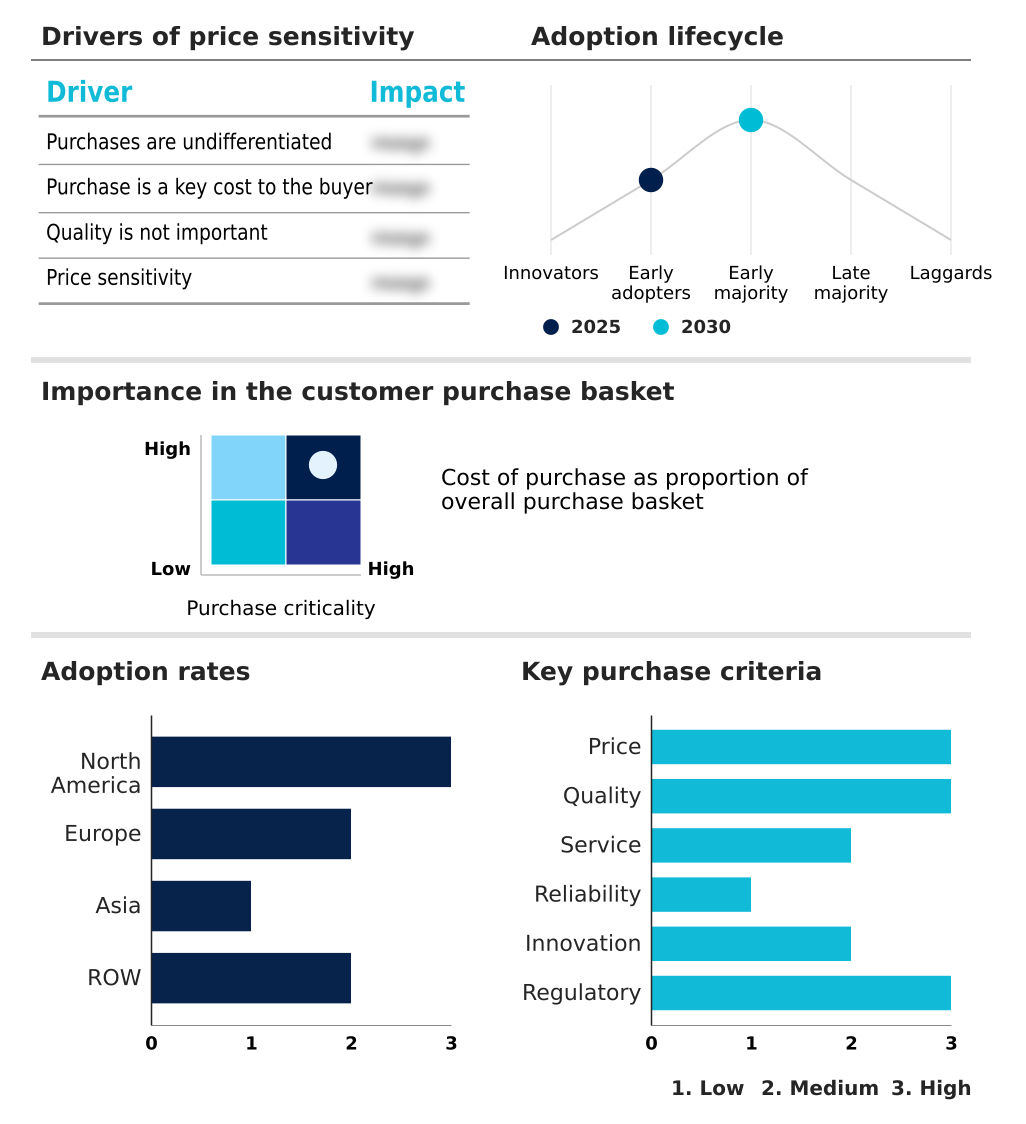
The guidewires market forecasting report includes the adoption lifecycle of the market, covering from the innovator’s stage to the laggard’s stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the guidewires market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape of Guidewires Industry
Competitive Landscape
Companies are implementing various strategies, such as strategic alliances, guidewires market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
Abbott Laboratories - Emphasizes balanced torque control, support, and flexibility in guidewires for complex coronary and peripheral procedures, spanning both workhorse and specialty lines.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Abbott Laboratories
- Amecath
- AngioDynamics Inc.
- Asahi Intecc Co. Ltd.
- B.Braun SE
- Becton Dickinson and Co.
- BIOTRONIK SE and Co. KG
- Boston Scientific Corp.
- Cardinal Health Inc.
- Cook Group Inc.
- Cryolife Inc.
- Lepu Medical Technology
- Medtronic Plc
- Merit Medical Systems Inc.
- Rocamed
- Rontis Corp. SA
- Stryker Corp.
- Teleflex Inc.
- Terumo Corp.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Guidewires market
- In October 2024, Medtronic Plc announced the launch of its new Sphere-14 neurovascular guidewire, engineered with advanced composite cores for enhanced trackability in treating ischemic stroke.
- In January 2025, Boston Scientific Corp. received FDA approval for its Victory-CTO guidewire, a specialized device with a high tip load designed for penetrating chronic total occlusions in coronary arteries.
- In March 2025, Abbott Laboratories completed its acquisition of VascPath Inc., a startup focused on innovative polymer jackets for peripheral guidewires, strengthening its portfolio for below-the-knee interventions.
- In May 2025, Terumo Corp. entered into a strategic partnership with NanoCoat Solutions to co-develop a next-generation durable hydrophilic coating, aiming to reduce delamination risk across its interventional product lines.
Dive into Technavio’s robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Guidewires Market insights. See full methodology.
| Market Scope | |
|---|---|
| Page number | 306 |
| Base year | 2025 |
| Historic period | 2020-2024 |
| Forecast period | 2026-2030 |
| Growth momentum & CAGR | Accelerate at a CAGR of 6.4% |
| Market growth 2026-2030 | USD 951.9 million |
| Market structure | Fragmented |
| YoY growth 2025-2026(%) | 6.1% |
| Key countries | US, Canada, Mexico, Germany, UK, France, Italy, The Netherlands, Spain, Russia, China, Japan, India, South Korea, Indonesia, Thailand, Singapore, Australia, UAE, Brazil, South Africa, Saudi Arabia and Turkey |
| Competitive landscape | Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
Research Analyst Overview
- The guidewires market is defined by continuous technical advancement, where core-to-tip design and guidewire stiffness are meticulously engineered to enhance procedural outcomes. The use of a nitinol core for kink resistance and superior tip flexibility is becoming standard, especially in neurovascular intervention where navigating vessel tortuosity is critical.
- For percutaneous coronary intervention, particularly in a heavily calcified lesion or chronic total occlusion, guidewire pushability and lesion crossing support are paramount. This has led to the development of composite cores and specialized atraumatic tip designs with high tip load.
- Advanced polymer jacket and hydrophilic coating technologies significantly improve lubricity and guidewire trackability, reducing friction and enabling smoother passage of devices like a drug-coated balloon or a distal access catheter. The adoption of catheter based technology, such as a radial PTA dilatation catheter or a thrombectomy aspiration system for mechanical thrombectomy, is entirely dependent on guidewire compatibility.
- For boardroom-level strategy, demonstrating a higher procedural success rate, such as a 10% improvement in first-pass success in complex cases, is key to justifying premium pricing.
- Innovations like enhanced tip shape retention and designs that enable the renal denervation system are expanding the applications for this foundational endovascular procedure tool, ensuring a high rate of embolic protection and overall procedural safety.
What are the Key Data Covered in this Guidewires Market Research and Growth Report?
-
What is the expected growth of the Guidewires Market between 2026 and 2030?
-
USD 951.9 million, at a CAGR of 6.4%
-
-
What segmentation does the market report cover?
-
The report is segmented by Product (Coronary guidewires, Peripheral guidewires, Urology guidewires, and Neurovascular), End-user (Cardiac catheterization laboratories, Hospitals, Specialty clinics, and Ambulatory surgical centers), Type (Hydrophilic, Uncoated, and Hydrophobic) and Geography (North America, Europe, Asia, Rest of World (ROW))
-
-
Which regions are analyzed in the report?
-
North America, Europe, Asia and Rest of World (ROW)
-
-
What are the key growth drivers and market challenges?
-
Escalating global burden of chronic and age related diseases, Intensifying pricing pressure and threat of commoditization
-
-
Who are the major players in the Guidewires Market?
-
Abbott Laboratories, Amecath, AngioDynamics Inc., Asahi Intecc Co. Ltd., B.Braun SE, Becton Dickinson and Co., BIOTRONIK SE and Co. KG, Boston Scientific Corp., Cardinal Health Inc., Cook Group Inc., Cryolife Inc., Lepu Medical Technology, Medtronic Plc, Merit Medical Systems Inc., Rocamed, Rontis Corp. SA, Stryker Corp., Teleflex Inc. and Terumo Corp.
-
Market Research Insights
- Market dynamics are increasingly influenced by the shift to value based healthcare, which prioritizes the total cost of an episode of care over individual component costs. This trend favors procedural solution offerings that can demonstrate improved procedural efficiency, such as reducing intervention times by 15% compared to multi-vendor systems.
- The migration of procedures to lower-cost outpatient settings, including ambulatory surgical centers and office based labs, is accelerating, with these sites seeing a 20% higher adoption rate of versatile, cost-effective guidewires. Procurement is also evolving, with group purchasing organizations consolidating buying power, impacting pricing strategies.
- Consequently, manufacturers must provide extensive clinical evidence to support premium technologies and adapt sales models to engage directly with physician-owners in these alternative sites of care.
We can help! Our analysts can customize this guidewires market research report to meet your requirements.