Pharmaceutical Contract Manufacturing Market Size 2025-2029
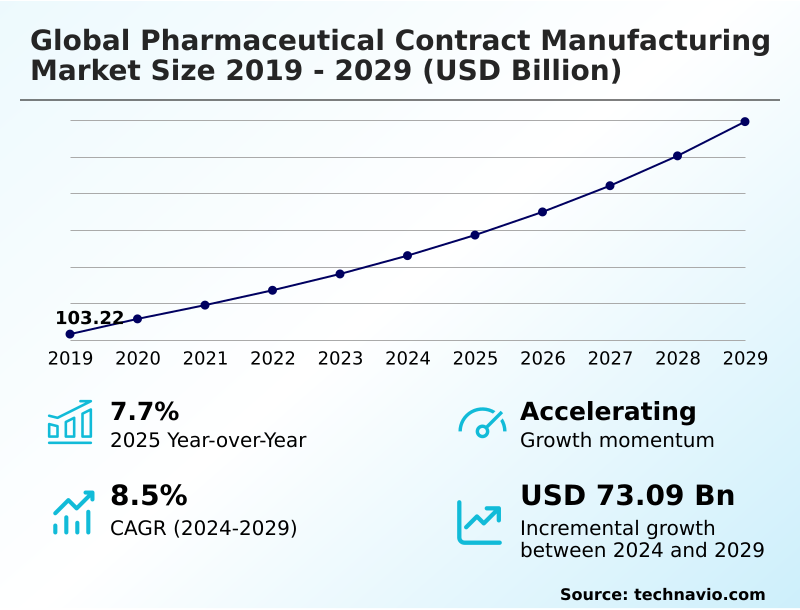
The pharmaceutical contract manufacturing market size is valued to increase by USD 73.09 billion, at a CAGR of 8.5% from 2024 to 2029. Increasing complexity and cost of research and development will drive the pharmaceutical contract manufacturing market.
Major Market Trends & Insights
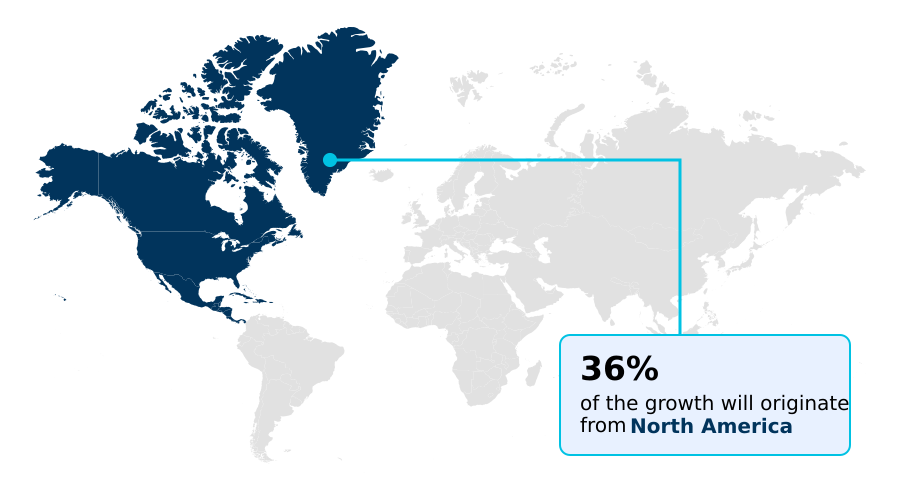
- North America dominated the market and accounted for a 36.1% growth during the forecast period.
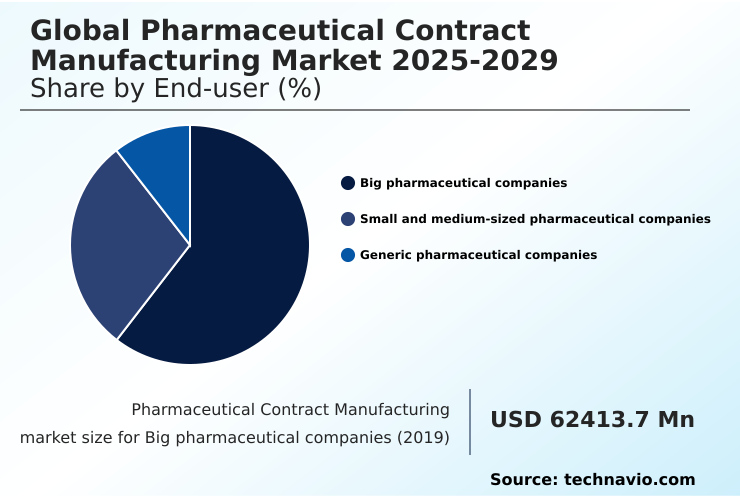
- By End-user - Big pharmaceutical companies segment was valued at USD 80.37 billion in 2023
- By Service - API and bulk drug manufacturing segment accounted for the largest market revenue share in 2023
Market Size & Forecast
- Market Opportunities: USD 115.89 billion
- Market Future Opportunities: USD 73.09 billion
- CAGR from 2024 to 2029 : 8.5%
Market Summary
- The pharmaceutical contract manufacturing market is undergoing a significant transformation, driven by the dual pressures of increasing drug development complexity and the imperative for cost optimization. Pharmaceutical companies are increasingly relying on contract partners to manage intricate manufacturing processes, particularly for biologics and advanced therapies, which require specialized expertise and substantial capital investment that is often unfeasible to maintain in-house.
- This strategic shift allows drug developers to focus on their core competencies in research and commercialization. A key trend is the move toward end-to-end service providers that can manage a product's entire journey from clinical development to commercial launch.
- For instance, a mid-sized biotech firm can partner with a single contract organization to handle everything from process development and manufacturing of clinical trial materials to regulatory submissions and final packaging, streamlining operations and accelerating time-to-market.
- However, the industry faces challenges related to maintaining quality control across a decentralized supply chain and navigating the complex global regulatory landscape, which demands rigorous compliance and validation at every stage.
What will be the Size of the Pharmaceutical Contract Manufacturing Market during the forecast period?
Get Key Insights on Market Forecast (PDF) Request Free Sample
How is the Pharmaceutical Contract Manufacturing Market Segmented?
The pharmaceutical contract manufacturing industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2025-2029, as well as historical data from 2019-2023 for the following segments.
- End-user
- Big pharmaceutical companies
- Small and medium-sized pharmaceutical companies
- Generic pharmaceutical companies
- Service
- API and bulk drug manufacturing
- Final dosage form
- Secondary packaging
- Application
- Infectious diseases
- Cardiovascular diseases
- Oncology
- Geography
- North America
- US
- Canada
- Mexico
- Asia
- Europe
- Germany
- UK
- France
- Rest of World (ROW)
- North America
By End-user Insights
The big pharmaceutical companies segment is estimated to witness significant growth during the forecast period.
The pharmaceutical contract manufacturing market segment for large companies is shaped by strategic outsourcing to enhance operational efficiency. These companies leverage external expertise for complex processes, including active pharmaceutical ingredient (api) synthesis and final dosage form manufacturing.
The increasing prevalence of biologics drives demand for specialized monoclonal antibody production and bioprocessing optimization. Outsourced pharmaceutical manufacturing allows large firms to focus on core competencies.
A key aspect of pharmaceutical outsourcing trends is managing the entire lifecycle, from clinical trial material manufacturing to commercial supply. Effective supply chain management and secondary packaging solutions provided by a contract development and manufacturing organization (cdmo) are critical.
This biopharmaceutical contract manufacturing model supports clinical to commercial manufacturing, with effective pharmaceutical supply chain outsourcing leading to up to a 15% reduction in logistical overhead.
The Big pharmaceutical companies segment was valued at USD 80.37 billion in 2023 and showed a gradual increase during the forecast period.
Regional Analysis
North America is estimated to contribute 36.1% to the growth of the global market during the forecast period.Technavio’s analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
See How Pharmaceutical Contract Manufacturing Market Demand is Rising in North America Request Free Sample
The geographic landscape of the pharmaceutical contract manufacturing market is characterized by distinct regional strengths, with North America holding over 36% of the market share, driven by its advanced R&D ecosystem.
The region's focus is on high-value services like commercial scale-up and complex drug substance manufacturing.
Meanwhile, Asia, projected to grow at a rate nearly 0.5% higher than North America, is a hub for cost-effective production and is rapidly advancing its technical capabilities in areas like microbial fermentation and pre-formulation development.
European providers are noted for their stringent adherence to quality standards, such as ICH Q7 guidelines, and expertise in formulation development services.
The adoption of a virtual pharma manufacturing model is encouraging a globalized API sourcing strategy, where technology transfer services and integrated cdmo services are crucial.
This dynamic allows companies to leverage regional specializations, with bioanalytical services outsourcing and pharmaceutical packaging outsourcing showing efficiency gains of over 15%.
Market Dynamics
Our researchers analyzed the data with 2024 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
- Strategic decision-making in the pharmaceutical contract manufacturing market requires a granular approach. When comparing cdmos for sterile injectable manufacturing, firms must assess capabilities beyond basic production. The cost analysis of outsourcing api manufacturing must account for long-term supply chain resilience with dual sourcing cdmos, a strategy that mitigates geopolitical and logistical risks.
- Navigating the challenges in technology transfer to a cdmo is critical, especially for complex biologics. The regulatory pathways for outsourced biologic drugs demand deep expertise, which influences the cdmo partnership for commercial scale-up. As firms select a cdmo for cell therapy production, they prioritize partners with proven track records in this nascent field.
- The role of cdmos in orphan drug development is expanding, as smaller patient populations necessitate flexible and scalable manufacturing solutions. Increasingly, the benefits of integrated cdmo service models are clear, offering streamlined project management. Trends in pharmaceutical serialization outsourcing are driven by global track-and-trace mandates.
- Managing intellectual property with a cdmo partner requires robust legal frameworks, especially as cdmo capabilities for antibody-drug conjugates advance. Organizations are rigorously evaluating cdmo quality management systems. The small molecule vs large molecule cdmo selection depends heavily on the product pipeline. The impact of continuous manufacturing on cdmos is reshaping facility design.
- Expertise in cdmo services for clinical trial supply chains ensures trials are not delayed. Outsourcing fill-finish for biologic products and understanding the geographic considerations for cdmo selection are pivotal. Finally, navigating cdmo contracts and agreements and implementing risk mitigation in pharmaceutical outsourcing are foundational to success, with comprehensive agreements reducing compliance-related delays by over 20%.
What are the key market drivers leading to the rise in the adoption of Pharmaceutical Contract Manufacturing Industry?
- The escalating complexity and substantial cost associated with pharmaceutical research and development serve as a primary market driver.
- Market growth is fundamentally driven by the need for specialized capabilities and capital efficiency. The complexity of modern therapeutics, such as those requiring viral vector production or plasmid dna manufacturing, necessitates pharma manufacturing partnerships with expert providers.
- The development of high-potency active pharmaceutical ingredients (hpapi) and antibody-drug conjugate (adc) development requires controlled environments that many companies cannot build internally.
- Outsourcing provides cost-efficient drug production, as adopting continuous manufacturing processes has demonstrated the potential to shrink facility footprints by 70%.
- Furthermore, regulatory compliance outsourcing is a major driver, with partners ensuring cgmp compliance services and maintaining high standards of quality control in contract manufacturing.
- This enables access to flexible manufacturing platforms and facilitates cdmo capacity expansion without direct capital expenditure on small molecule synthesis facilities, reducing investment risk by over 50%.
What are the market trends shaping the Pharmaceutical Contract Manufacturing Industry?
- Intensifying cost-containment pressures and the persistent pursuit of operational efficiency are defining the market. These factors compel strategic shifts in manufacturing and supply chain management.
- Key trends in the market are centered on advanced manufacturing technologies and specialized services. The demand for biologics manufacturing is fueling investments in end-to-end manufacturing solutions for large molecule manufacturing. This includes a focus on sterile fill-finish services and lyophilization services, which are critical for parenteral drugs.
- The rise of advanced therapy medicinal products (atmp) has intensified the need for specialized cdmo services in cell and gene therapy manufacturing. Expertise in aseptic processing techniques is paramount, with automation reducing human-error contamination events by over 40%.
- As supply chains become more complex, efficient tech transfer in pharma, robust serialization and aggregation systems, and sophisticated cold chain logistics are essential. Firms leveraging advanced cold chain logistics have reported a 99.8% product integrity rate. The market is also seeing growth in capabilities for small molecule cdmo development.
What challenges does the Pharmaceutical Contract Manufacturing Industry face during its growth?
- Heightened regulatory scrutiny and increasing complexity present a significant challenge to industry growth and operational stability.
- Navigating the market involves significant challenges, primarily centered on regulatory complexity and quality assurance. Stringent cdmo selection criteria are necessary to ensure partners can meet evolving standards. Firms require robust quality assurance systems and comprehensive process validation services, which can shorten regulatory review cycles by 15% when implemented effectively.
- Managing regulatory affairs support for products like sterile injectable manufacturing or those involving controlled substance handling is a major operational burden. The technical demands of analytical testing services and long-term stability testing protocols add further complexity. For specialized drug product services, including solid-state characterization, finding a partner with the right expertise is crucial.
- Furthermore, process development outsourcing for commercial drug manufacturing requires seamless integration, while ensuring reliable clinical supply services remains a logistical hurdle. Effective quality systems can decrease batch rejection rates by over 50%.
Exclusive Technavio Analysis on Customer Landscape
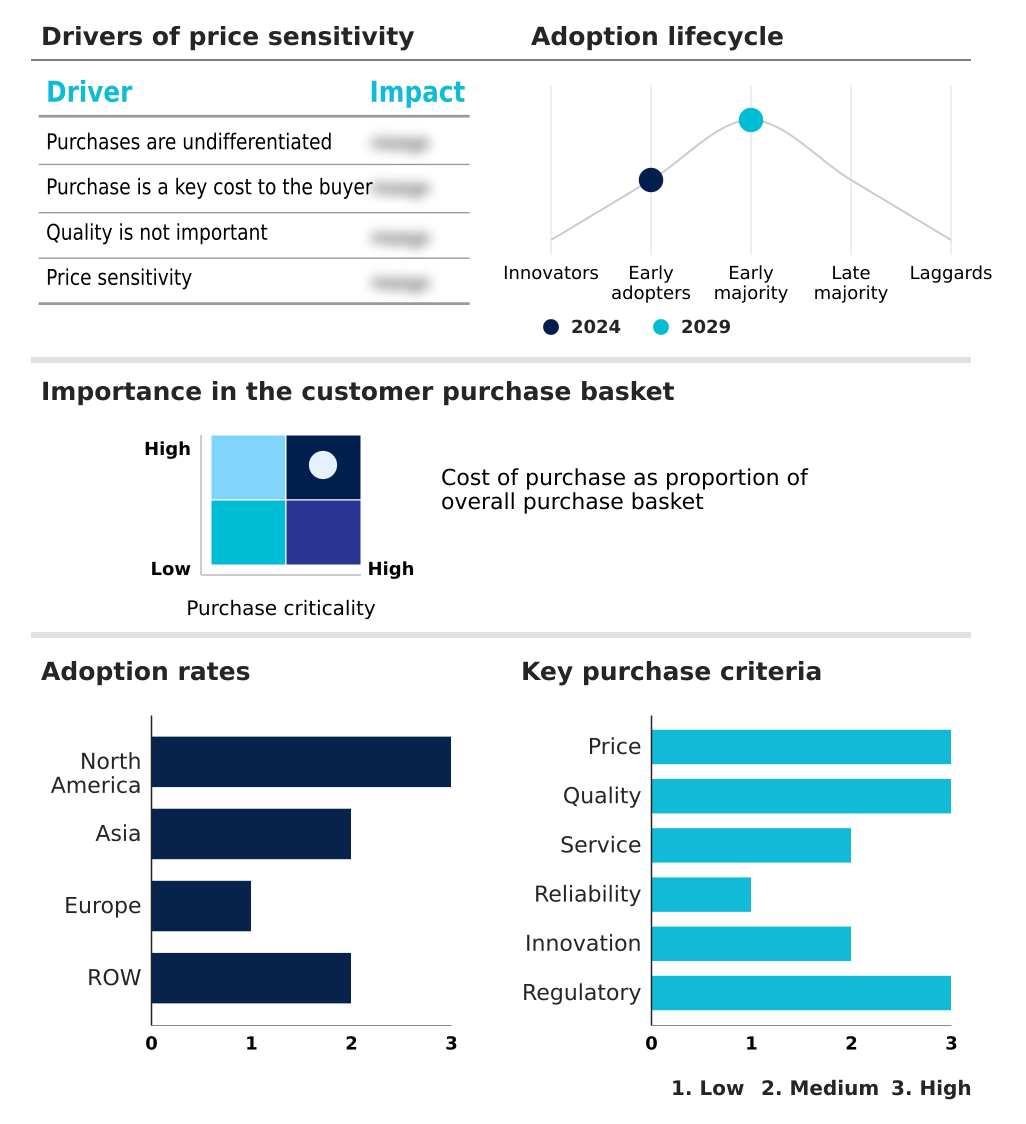
The pharmaceutical contract manufacturing market forecasting report includes the adoption lifecycle of the market, covering from the innovator’s stage to the laggard’s stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the pharmaceutical contract manufacturing market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape of Pharmaceutical Contract Manufacturing Industry
Competitive Landscape
Companies are implementing various strategies, such as strategic alliances, pharmaceutical contract manufacturing market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
AbbVie Inc. - Provides integrated contract services, including API development and manufacturing, diagnostic solutions, and comprehensive pharmaceutical development support from early to late phase.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- AbbVie Inc.
- Almac Group Ltd.
- Baxter International Inc.
- Boehringer Ingelheim GmbH
- Cadila Pharmaceuticals Ltd.
- Charles River Laboratories
- Cmic Holdings Co. Ltd.
- Dalton Pharma Services
- Dr Reddys Laboratories Ltd.
- Grifols SA
- Laboratory Corp.
- Lonza Group Ltd.
- Lupin Ltd.
- Novotech Health Holdings
- OPTIMAPHARM d.o.o.
- Parexel International Corp.
- PCI Pharma Services
- Recipharm AB
- Syneos Health
- Thermo Fisher Scientific Inc.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Pharmaceutical contract manufacturing market
- In May 2025, Sanofi announced its intention to invest at least $20 billion in the United States through 2030 to boost its manufacturing and research capabilities through direct investment and local collaborations.
- In May 2025, Bora Biologics announced an expansion of its San Diego facility to support the manufacturing of its growing clinical and commercial pipeline.
- In April 2025, Samsung Biologics is scheduled to complete its fifth manufacturing plant, a move aimed at significantly increasing its total biopharmaceutical production capacity to meet global demand.
- In December 2024, Lonza Group Ltd. announced a major expansion of its cell and gene therapy manufacturing facilities to meet escalating demand for advanced therapy medicinal products.
Dive into Technavio’s robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Pharmaceutical Contract Manufacturing Market insights. See full methodology.
| Market Scope | |
|---|---|
| Page number | 293 |
| Base year | 2024 |
| Historic period | 2019-2023 |
| Forecast period | 2025-2029 |
| Growth momentum & CAGR | Accelerate at a CAGR of 8.5% |
| Market growth 2025-2029 | USD 73087.9 million |
| Market structure | Fragmented |
| YoY growth 2024-2025(%) | 7.7% |
| Key countries | US, Canada, Mexico, China, India, Japan, South Korea, Indonesia, Thailand, Germany, UK, France, Italy, Spain, The Netherlands, Brazil, Saudi Arabia, UAE, Turkey, Argentina, Colombia, South Africa and Israel |
| Competitive landscape | Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
Research Analyst Overview
- The pharmaceutical contract manufacturing market provides a critical infrastructure supporting the entire drug development lifecycle. It begins with pre-formulation and formulation development services, including solid-state characterization, moving into small molecule synthesis or more complex biologics manufacturing. For advanced therapies, expertise in viral vector production, plasmid dna manufacturing, and cell and gene therapy manufacturing is essential.
- As molecules progress, contract providers manage active pharmaceutical ingredient (api) synthesis, including high-potency active pharmaceutical ingredients (hpapi), and drug substance manufacturing through microbial fermentation or other bioprocessing optimization techniques. A key focus is on monoclonal antibody production and antibody-drug conjugate (adc) development. Manufacturing of clinical trial material manufacturing transitions to commercial scale-up, often using continuous manufacturing processes.
- Final production stages involve aseptic processing techniques for sterile fill-finish services, lyophilization services, and final dosage form manufacturing. Throughout, cgmp compliance services, robust quality assurance systems, process validation services, and adherence to ICH Q7 guidelines are non-negotiable. Services like analytical testing services and stability testing protocols ensure product integrity.
- Firms offering regulatory affairs support for controlled substance handling and providing technology transfer services are invaluable. Comprehensive supply chain management, including secondary packaging solutions, serialization and aggregation, and cold chain logistics, completes the end-to-end offering, with integrated partners improving supply chain efficiency by up to 25%.
What are the Key Data Covered in this Pharmaceutical Contract Manufacturing Market Research and Growth Report?
-
What is the expected growth of the Pharmaceutical Contract Manufacturing Market between 2025 and 2029?
-
USD 73.09 billion, at a CAGR of 8.5%
-
-
What segmentation does the market report cover?
-
The report is segmented by End-user (Big pharmaceutical companies, Small and medium-sized pharmaceutical companies, and Generic pharmaceutical companies), Service (API and bulk drug manufacturing, Final dosage form, and Secondary packaging), Application (Infectious diseases, Cardiovascular diseases, and Oncology) and Geography (North America, Asia, Europe, Rest of World (ROW))
-
-
Which regions are analyzed in the report?
-
North America, Asia, Europe and Rest of World (ROW)
-
-
What are the key growth drivers and market challenges?
-
Increasing complexity and cost of research and development, Heightened regulatory scrutiny and complexity
-
-
Who are the major players in the Pharmaceutical Contract Manufacturing Market?
-
AbbVie Inc., Almac Group Ltd., Baxter International Inc., Boehringer Ingelheim GmbH, Cadila Pharmaceuticals Ltd., Charles River Laboratories, Cmic Holdings Co. Ltd., Dalton Pharma Services, Dr Reddys Laboratories Ltd., Grifols SA, Laboratory Corp., Lonza Group Ltd., Lupin Ltd., Novotech Health Holdings, OPTIMAPHARM d.o.o., Parexel International Corp., PCI Pharma Services, Recipharm AB, Syneos Health and Thermo Fisher Scientific Inc.
-
Market Research Insights
- The pharmaceutical contract manufacturing market is defined by a shift toward strategic partnerships. The outsourced pharmaceutical manufacturing model, leveraging a contract development and manufacturing organization (cdmo) or a contract manufacturing organization (cmo), is central to modern pharmaceutical outsourcing trends. Companies seek end-to-end manufacturing solutions for both large molecule manufacturing and small molecule cdmo services, including advanced therapy medicinal products (atmp).
- Successful tech transfer in pharma, supported by cdmo capacity expansion, is critical. These pharma manufacturing partnerships are driven by the need for regulatory compliance outsourcing and cost-efficient drug production. Adopting flexible manufacturing platforms improves quality control in contract manufacturing by over 30%.
- The demand for sterile injectable manufacturing and comprehensive drug product services, including process development outsourcing and clinical supply services for commercial drug manufacturing, is high. Proper cdmo selection criteria and integrated cdmo services are key for the virtual pharma manufacturing model, with bioanalytical services outsourcing and strategic pharmaceutical packaging outsourcing and api sourcing strategy reducing time-to-market by up to 20%.
We can help! Our analysts can customize this pharmaceutical contract manufacturing market research report to meet your requirements.