Protein Therapeutics Market Size 2024-2028
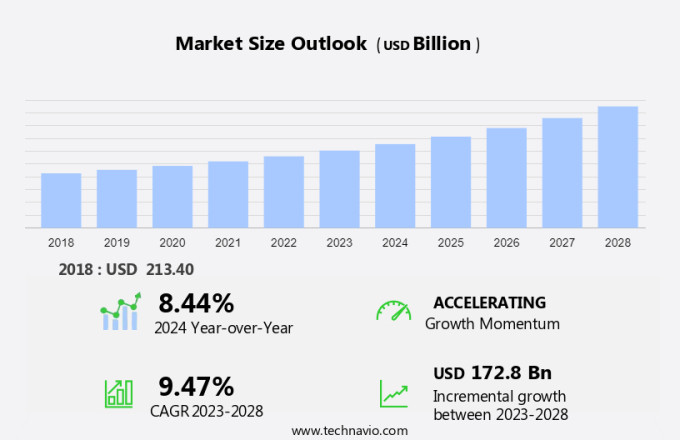
The protein therapeutics market size is forecast to increase by USD 172.8 billion at a CAGR of 9.47% between 2023 and 2028. The market is experiencing significant growth due to the increased demand for monoclonal antibodies (mAbs) and the continued focus on improving the efficacy and safety of these therapeutics. Companies such as Autolus and Lumosa Therapeutics are at the forefront of this innovation, developing genetically engineered versions of human proteins for disease treatment. However, the market faces challenges including the complexities in manufacturing, storage conditions, distribution policies, and high costs. For instance, bioscience reagents like PeproTech provide essential tools for research and development in the field, but their use in therapeutic protein drugs adds to the overall cost. Moreover, the protein's activity duration and the need for sugar molecules to maintain stability further increase the complexity and cost. Furthermore, despite these challenges, the market for therapeutic protein drugs continues to grow, with applications in immunological diseases, hematological disorders, and chemotherapy. Genetically engineered proteins and natural proteins both play crucial roles in medicine, offering potential solutions to various disease conditions.
The market is a significant segment of the medicines and treatments industry, focusing on the development and production of therapeutic protein drugs for various chronic diseases. These conditions include cancer, diabetes, autoimmune disorders, cardiovascular diseases, and others. The market encompasses both genetically engineered proteins and natural proteins, derived from sugars and molecules, to mimic the body's natural response. Therapeutic protein drugs offer versatility and effectiveness, particularly in treating immunological diseases, hematological disorders, and metabolic disorders such as diabetes. The monoclonal antibodies segment holds a substantial market share due to their long protein activity duration and potential for targeted therapy.
Furthermore, major healthcare institutions and pharmaceutical companies invest heavily in research and development to create quality drugs that cater to the growing demand for effective treatments. Developing economies are increasingly contributing to the protein therapy market's growth due to the rising prevalence of chronic disorders and improving healthcare infrastructure. Chemotherapy and other traditional treatments continue to face challenges in terms of side effects and limited efficacy, making protein therapeutics a promising alternative for various diseases. Recombinant proteins are gaining popularity due to their ability to produce consistent, high-quality therapeutic agents.
Market Segmentation
The market research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD billion" for the period 2024-2028, as well as historical data from 2018-2022 for the following segments.
- Product
- mAbs
- Human insulin
- Erythropoeitin
- Clotting factors
- Others
- Application
- Metabolic disorders
- Immunologic disorders
- Hematological disorders
- Cancer
- Genetic disorders
- Geography
- North America
- Canada
- US
- Europe
- Germany
- UK
- Asia
- China
- Rest of World (ROW)
- North America
By Product Insights
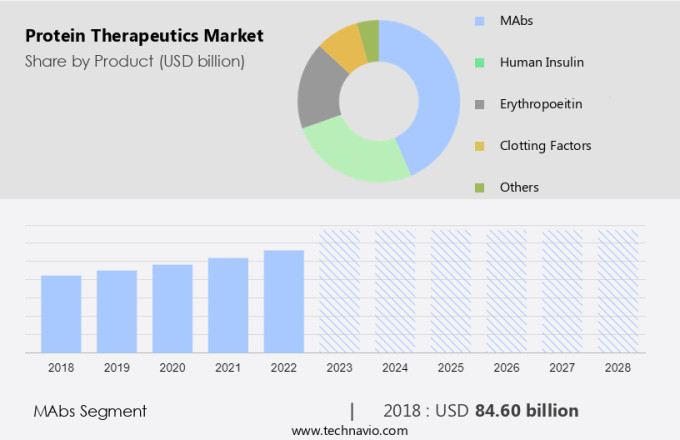
The MAbs segment is estimated to witness significant growth during the forecast period. Protein therapeutics, including genetically engineered versions of human proteins and natural proteins, are gaining significant attention in the medical field due to their effectiveness in treating various diseases, particularly cancer and immunological diseases. The increasing burden of cancer, caused by lifestyle factors such as smoking, alcohol consumption, and sedentary work, has led to a rise in the demand for protein therapeutics. Monoclonal antibodies (mAbs), such as GAZYVA, BLINCYTO, Removab, and POTELIGEO, are major next-generation antibodies used to treat cancer. These mAbs are often linked with cytotoxic drugs, such as Antibody-Drug Conjugates (ADCs), to enhance their potency and efficiency. ADCs, such as Seattle Genetics' SGN-35, ImmunoGen's IMGN110, and Immunomedics' IMMU-132, have shown promising results in treating various diseases, including cancers and hematological malignancies.
In addition, the increasing use of ADCs in drug development is expected to boost revenue generation in The market. Additionally, the market for bioscience reagents, including therapeutic proteins and their related molecules, is expected to grow due to the rising demand for protein replacement therapy and the development of novel technologies. PeproTech, a leading supplier of high-quality proteins and antibodies, plays a crucial role in the production of these essential reagents. Overall, the market is expected to continue its growth trajectory, driven by the increasing incidence of diseases and the development of novel protein-based therapeutics.
Get a glance at the market share of various segments Request Free Sample
The MAbs segment accounted for USD 84.60 billion in 2018 and showed a gradual increase during the forecast period.
Regional Insights
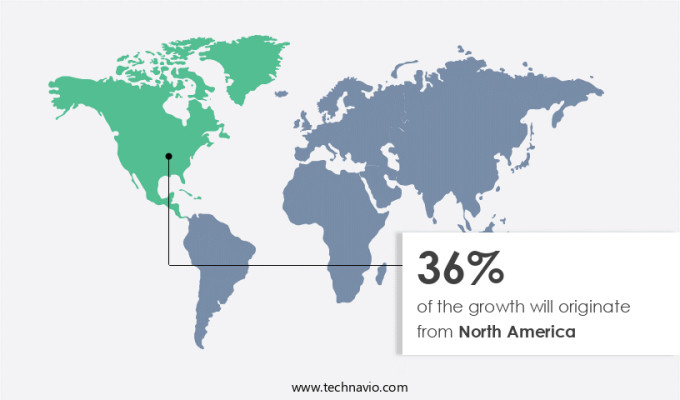
North America is estimated to contribute 36% to the growth of the global market during the forecast period. Technavio's analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
For more insights on the market share of various regions Request Free Sample
The market in North America is experiencing significant growth due to the increasing adoption of existing protein therapeutics and the introduction of new ones. For instance, Novartis introduced Cosentyx for the treatment of autoimmune and inflammatory diseases, while Bristol-Myers Squibb launched OPDIVO (nivolumab) for advanced renal cell carcinoma and received US FDA approval for its use in treating Hodgkin lymphoma. Taltz (ixekizumab) was approved by the US FDA for psoriasis treatment, demonstrating significant skin clearance in 87%-90% of patients in clinical trials. Pembrolizumab (KEYTRUDA) was approved for unresectable or metastatic melanoma, elotuzumab (Empliciti) for multiple myeloma, and necitumumab (Portrazza) for metastatic squamous NSCLC.
Moreover, companies such as Autolus and Lumosa Therapeutics are also developing novel protein therapeutics for various diseases, including acute ischemic stroke and immunological diseases. Protein therapeutics, which include genetically engineered versions of human proteins and natural proteins, are used for protein replacement therapy, chemotherapy, and disease treatment. These therapeutic protein drugs are composed of sugars and molecules that enhance the protein's activity duration, making them effective medicines for various disorders.
Our researchers analyzed the data with 2023 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
Market Driver
Increased demand for mAbs is the key driver of the market. Monoclonal antibodies (mAbs) are a significant segment of The market, driven by the increasing understanding of diseases at the molecular level. These antibodies exhibit a high level of specificity towards disease cells and targeted areas, making them an effective tool in various therapies such as radioimmunotherapy and antibody-directed enzyme prodrug therapy. MAbs are utilized in the development of biopharmaceutical drugs for treating various diseases, including cancer, rheumatoid arthritis (RA), systemic lupus erythematosus, multiple sclerosis, Crohn's disease, psoriasis, ulcerative colitis, transplantation, insulin for diabetic cases, hormone replacement, and metabolic disorders like Gaucher's disease and hemochromatosis.
The growing application of mAbs in drug development contributes to the market's expansion, with ongoing research in immunological disorders, chemotherapy treatment for cancer, and biologics for AIDS further fueling the growth. Furthermore, BioNtech, among other leading companies, is at the forefront of this innovation, driving advancements in protein therapeutics.
Market Trends
Continued focus on improvement of protein therapeutics is the upcoming trend in the market. Protein therapeutics have gained significant attention in the healthcare industry due to their potential applications in managing various medical conditions, including rheumatoid arthritis, cancers, systemic lupus erythematosus, multiple sclerosis, Crohn's disease, psoriasis, ulcerative colitis, and metabolic disorders such as diabetes. These therapies offer targeted treatment approaches for patients, addressing specific protein deficiencies or overexpressions. For instance, in diabetes, insulin and hormones that regulate glucose metabolism are crucial. Protein therapeutics can also be used for transplantation and chemotherapy treatment in cancer patients, as well as for immunological disorders like AIDS. Companies like BioNtech are at the forefront of innovation, developing advanced protein therapeutics.
Moreover, companies are focusing on launching improved products with unique features to cater to diverse patient needs. For example, LINET and LINAK offer four-section profile beds with knee raises and ultra-low beds. These beds provide better comfort and safety, with the former offering supported sitting positions with less abdominal compression and the latter enabling custom height entry/exit positions for patients with mobility restrictions.
Market Challenge
Complexities in manufacturing, storage conditions, distribution policies, and high cost is a key challenge affecting market growth. Protein therapeutics have gained significant attention in the medical industry due to their potential in treating various conditions, including rheumatoid arthritis, cancers, systemic lupus erythematosus, multiple sclerosis, Crohn's disease, psoriasis, ulcerative colitis, transplantation, metabolic disorders such as diabetes, and immunological disorders. These complex biologics are produced from living organisms, making their manufacturing process intricate and time-consuming. The process involves fermentation, clarification, separation, and purification. The separation and purification stages are particularly challenging due to the subtle differences between various protein molecules. HPLC and spectroscopy are essential analytical methods to ensure product quality. Maintaining proper temperature, pressure, and pH throughout the process is crucial.
For instance, the structural differences between Eprex, a biosimilar, and Amgen's EPOGEN led to instability issues. Proteins are also used in chemotherapy treatments for cancer and in addressing conditions like Gaucher's disease, hemochromatosis, and AIDS. BioNtech, a leading player in protein therapeutics, is pioneering innovative solutions, further expanding the market's potential.
Exclusive Customer Landscape
The market forecasting report includes the adoption lifecycle of the market, covering from the innovator's stage to the laggard's stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape
Key Companies & Market Insights
Companies are implementing various strategies, such as strategic alliances, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the market.
Amgen Inc.- The company offers and focuses on discovering, developing, manufacturing, and delivering innovative human therapeutics.
The market research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- AbbVie Inc.
- AstraZeneca Plc
- Biocon Ltd.
- Biogen Inc.
- Bristol Myers Squibb Co.
- Celltrion Healthcare Co. Ltd.
- Creative Biolabs
- Eli Lilly and Co.
- F. Hoffmann La Roche Ltd.
- Intas Pharmaceuticals Ltd.
- Ipsen Pharma
- Johnson and Johnson Services Inc.
- LGM Pharma LLC
- Midas Pharma GmbH
- Novartis AG
- Novo Nordisk AS
- Pfizer Inc.
- PV Pharma Healthcare Pvt. Ltd.
- Sanofi SA
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key market players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Research Analyst Overview
Protein therapeutics, a branch of medicines that utilizes innovative biological drugs derived from human proteins, play a significant role in treating various chronic diseases, including cancer, diabetes, autoimmune disorders, cardiovascular diseases, and others. Major healthcare institutions and pharmaceutical firms collaborate with research centers, academic institutions, and biotechnology companies to develop protein-based drugs using advanced techniques such as recombinant DNA technology, protein engineering, and cell culture techniques. The rising prevalence of chronic diseases, particularly among the geriatric population, fuels the demand for protein therapeutics. These therapies offer improved patient outcomes, making them a valuable investment for healthcare expenditure and infrastructure.
In addition, technological advancements in protein synthesis, formulation techniques, and therapeutic protein drugs have led to the development of high-cost, complex procedures for treating diseases like rheumatoid arthritis, cancers, systemic lupus erythematosus, multiple sclerosis, Crohn's disease, psoriasis, ulcerative colitis, transplantation, and various metabolic disorders, including diabetes and hormonal disorders. Protein therapeutics include monoclonal antibodies, insulin, hormones, and genetically engineered proteins, among others. The market for protein therapeutics is segmented based on applications, including immunologic disorders, chronic disorders, and metabolic disorders. The application segments are further divided into monoclonal antibodies, fusion proteins, and other protein-based drugs. The market's growth is driven by the increasing prevalence of diseases, the development of versatile therapies, and the regulatory framework that supports the production and distribution of quality drugs.
Furthermore, the protein therapy market is expected to grow significantly due to the pipeline analysis of new drugs, the prevalence of diseases, and the increasing investment in research and development. The market report provides quantitative units and volumes for the base year and historic years, with a focus on the report metric, regulatory framework, and product segmentation. The market's growth is influenced by the high cost and complex procedures involved in producing these therapies, as well as the technological advancements that aim to improve patient epidemiology and therapeutic outcomes.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
191 |
|
Base year |
2023 |
|
Historic period |
2018-2022 |
|
Forecast period |
2024-2028 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 9.47% |
|
Market Growth 2024-2028 |
USD 172.8 billion |
|
Market structure |
Fragmented |
|
YoY growth 2023-2024(%) |
8.44 |
|
Regional analysis |
North America, Europe, Asia, and Rest of World (ROW) |
|
Performing market contribution |
North America at 36% |
|
Key countries |
US, Canada, Germany, UK, and China |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
|
Key companies profiled |
AbbVie Inc., Amgen Inc., AstraZeneca Plc, Biocon Ltd., Biogen Inc., Bristol Myers Squibb Co., Celltrion Healthcare Co. Ltd., Creative Biolabs, Eli Lilly and Co., F. Hoffmann La Roche Ltd., Intas Pharmaceuticals Ltd., Ipsen Pharma, Johnson and Johnson Services Inc., LGM Pharma LLC, Midas Pharma GmbH, Novartis AG, Novo Nordisk AS, Pfizer Inc., PV Pharma Healthcare Pvt. Ltd., and Sanofi SA |
|
Market dynamics |
Parent market analysis, market growth inducers and obstacles, market forecast, fast-growing and slow-growing segment analysis, COVID-19 impact and recovery analysis and future consumer dynamics, market condition analysis for the forecast period |
|
Customization purview |
If our market report has not included the data that you are looking for, you can reach out to our analysts and get segments customized. |
What are the Key Data Covered in this Market Research and Growth Report?
- CAGR of the market during the forecast period
- Detailed information on factors that will drive the market growth and forecasting between 2024 and 2028
- Precise estimation of the size of the market and its contribution of the market in focus to the parent market
- Accurate predictions about upcoming market growth and trends and changes in consumer behaviour
- Growth of the market across North America, Europe, Asia, and Rest of World (ROW)
- A thorough analysis of the market's competitive landscape and detailed information about companies
- Comprehensive analysis of factors that will challenge the growth of market companies
We can help! Our analysts can customize this market research report to meet your requirements. Get in touch