Biopharmaceutical Analytical Testing Services Market Size 2025-2029
The biopharmaceutical analytical testing services market size is valued to increase USD 5.48 billion, at a CAGR of 15.2% from 2024 to 2029. Increase in demand for mAbs will drive the biopharmaceutical analytical testing services market.
Major Market Trends & Insights
- Europe dominated the market and accounted for a 33% growth during the forecast period.
- By Service - Bioanalytical segment was valued at USD 1.09 billion in 2023
- By End-user - Pharma and biotech companies segment accounted for the largest market revenue share in 2023
Market Size & Forecast
- Market Opportunities: USD 266.87 million
- Market Future Opportunities: USD 5481.30 million
- CAGR : 15.2%
- Europe: Largest market in 2023
Market Summary
- The market encompasses a range of technologies and applications that play a crucial role in the development and production of biopharmaceuticals. Core technologies, such as chromatography and mass spectrometry, continue to evolve, driving advancements in areas like protein characterization and impurity analysis. Applications span from small molecule and peptide analysis to the increasing demand for monoclonal antibodies (mAbs), which account for over 60% of the total biologics market. Service types include contract testing services, in-house testing, and outsourced testing, with the outsourcing segment projected to grow due to the rise in investment in research and development.
- However, the market faces challenges such as the shortage of trained professionals, stringent regulations, and increasing competition. Despite these hurdles, the market presents significant opportunities, particularly in emerging regions like Asia Pacific, where the biopharmaceutical industry is rapidly expanding.
What will be the Size of the Biopharmaceutical Analytical Testing Services Market during the forecast period?
Get Key Insights on Market Forecast (PDF) Request Free Sample
How is the Biopharmaceutical Analytical Testing Services Market Segmented and what are the key trends of market segmentation?
The biopharmaceutical analytical testing services industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2025-2029, as well as historical data from 2019-2023 for the following segments.
- Service
- Bioanalytical
- Method development and validation
- Stability testing
- Others
- End-user
- Pharma and biotech companies
- CROs
- Type
- Physiochemical analysis
- Biological assays
- Microbial testing
- Environmental monitoring
- Geography
- North America
- US
- Canada
- Europe
- France
- Germany
- UK
- APAC
- China
- India
- Japan
- South Korea
- South America
- Brazil
- Rest of World (ROW)
- North America
By Service Insights
The bioanalytical segment is estimated to witness significant growth during the forecast period.
Bioanalytical testing plays a pivotal role in determining the quantitative properties of drugs and metabolites in various biological matrices, such as serum, blood, plasma, tissue, urine, and skin samples. Applications of these testing techniques span across various sectors, including bioavailability, pharmacology, bioequivalence, pharmacokinetic, and toxicology studies on humans and animals. The market is experiencing significant growth, with an estimated 30% of pharmaceutical companies outsourcing their bioanalytical testing needs to third-party laboratories. This trend is primarily driven by the increasing use of biosimilars for a range of medical indications, particularly diabetes and cancer. Moreover, the market is witnessing substantial investments from contract research organizations (CROs), pharmaceutical companies, and bioanalytical laboratories in expanding their bioanalytical services and establishing new testing facilities.
The Bioanalytical segment was valued at USD 1.09 billion in 2019 and showed a gradual increase during the forecast period.
According to industry reports, the market is projected to grow by 25% within the next five years. Bioanalytical testing encompasses a variety of methods and techniques, including microbial limits testing, sample preparation techniques, drug substance characterization, bioanalytical method validation, particle analysis techniques, protein quantification methods, pharmaceutical stability testing, analytical instrument calibration, mass spectrometry analysis, pharmaceutical testing automation, endotoxin detection methods, biopharmaceutical formulation testing, impurity profiling assays, bioavailability and pharmacokinetics, biopharmaceutical analytical instruments, advanced analytical techniques, method transfer protocols, immunogenicity assessment assays, and pharmaceutical regulatory compliance. Chromatographic separation techniques, sterility testing protocols, spectroscopic identification methods, reference standards qualification, and biopharmaceutical testing outsourcing are other essential aspects of the market.
These techniques and services ensure the quality, safety, and efficacy of pharmaceutical products, making them indispensable in the biopharmaceutical industry.
Regional Analysis
Europe is estimated to contribute 33% to the growth of the global market during the forecast period. Technavio's analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
See How Biopharmaceutical Analytical Testing Services Market Demand is Rising in Europe Request Free Sample
In North America, the market is the largest revenue generator, driven by the presence of leading companies like Charles River and Catalent. These firms offer a comprehensive range of services, from drug discovery to manufacturing, ensuring regulatory compliance for biopharmaceutical companies. In the US alone, over 500,000 people die annually from cancer, and around 610,000 from heart disease. Biopharmaceutical firms are dedicated to developing novel treatments for diseases with no current solutions.
To ensure safety, quality, and efficacy, these drugs undergo rigorous analytical testing. The market's continuous evolution reflects the pressing need for advanced testing techniques and the growing demand for personalized medicines.
Market Dynamics
Our researchers analyzed the data with 2024 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
The market encompasses a range of specialized techniques and methods to ensure the quality, safety, and efficacy of biopharmaceutical products. This market witnesses significant demand due to the increasing complexity of biotherapeutics and the stringent regulatory requirements for their approval. Key analytical techniques include HPLC method development for biopharmaceuticals, mass spectrometry for biopharmaceutical analysis, and biopharmaceutical impurity identification techniques. Pharmaceutical stability testing protocols and bioavailability studies using drug formulation are crucial to assess the longevity and efficacy of these products. Immunogenicity assessment in biotherapeutics and protein quantification using ELISA methods are essential to understand the potential adverse reactions and ensure the required therapeutic concentration.
Microbial limits testing for pharmaceutical products, endotoxin detection methods validation, sterility testing validation for pharmaceuticals, and particle analysis techniques for biopharmaceuticals are vital for maintaining product safety. In vitro drug release studies and biopharmaceutical analytical instrument calibration are essential for understanding the behavior of these complex molecules under various conditions. Data integrity in pharmaceutical testing and sample preparation techniques are crucial for accurate and reliable results. Reference standards qualification in biopharmaceuticals, method transfer protocols validation, and quality by design in biopharmaceutical testing are essential for maintaining consistency and efficiency in the testing process. The market for biopharmaceutical analytical testing services is highly competitive, with a minority of players accounting for a significantly larger share due to their expertise and advanced technology offerings.
For instance, the demand for advanced mass spectrometry techniques is nearly double that of HPLC method development in the market. This highlights the importance of staying updated with the latest analytical techniques and regulatory requirements to remain competitive in this market.
What are the key market drivers leading to the rise in the adoption of Biopharmaceutical Analytical Testing Services Industry?
- The significant rise in demand for monoclonal antibodies (mAbs) serves as the primary market driver.
- Monoclonal antibodies (mAbs) are proteins engineered in laboratories to bind specifically to antigens, making them essential therapeutic agents in the pharmaceutical and biotechnology industries. These antibodies, used individually or in combination with radiotherapy, drugs, or toxins, target and eliminate infected cells, particularly in cancer treatments. The market for monoclonal antibodies is expanding due to their application in immunotherapy, stimulating immune responses to combat diseases. Research and development for mAbs against various conditions, such as Ebola, rheumatoid arthritis, and cancers, is ongoing.
- Cancer drug-conjugated mAbs offer targeted treatments by cleaving at tumor sites. The growing demand for personalized medicine significantly contributes to the increased interest in monoclonal antibodies. The market's evolution reflects the continuous advancements in biotechnology and the ongoing efforts to address various health concerns.
What are the market trends shaping the Biopharmaceutical Analytical Testing Services Industry?
- The investment landscape is shifting towards an increase in research and development spending. This trend is set to define the market moving forward.
- The global research and development (R&D) spending is experiencing consistent growth, driven by the expanding economies of developing countries. This trend is leading to an increased number of laboratories and research facilities being established during the forecast period. In the pharmaceutical sector, the demand for analytical testing services is escalating, as these services are essential for the development of biopharmaceutical drugs. The expansion of facilities in drug discovery and pharmaceutical research sectors relies heavily on analytical testing services to ensure the production of safe and effective products.
- As innovation and knowledge expansion remain priorities for countries, the trend of increased R&D spending is expected to persist throughout the forecast period. This growth translates to the establishment of more facilities in various sectors, creating a significant demand for analytical testing services.
What challenges does the Biopharmaceutical Analytical Testing Services Industry face during its growth?
- The lack of adequately trained professionals poses a significant challenge to the industry's growth trajectory.
- In the dynamic biopharmaceutical industry, the demand for skilled laboratory professionals is paramount to ensure regulatory compliance and maintain product quality. According to a recent industry study, the non-compliance rate among laboratory workers hovers around 30%, a significant concern for manufacturing facilities and biotech companies alike. This non-compliance can lead to product failures, regulatory issues, and substantial financial losses. The root cause of this issue is the scarcity of adequately trained professionals. As a result, companies are investing in training programs and partnerships with educational institutions to address this skill gap.
- The ongoing evolution of testing technologies and regulatory requirements necessitates continuous learning and adaptation for laboratory personnel. This investment in human capital not only ensures regulatory compliance but also fosters innovation and efficiency within the industry.
Exclusive Customer Landscape
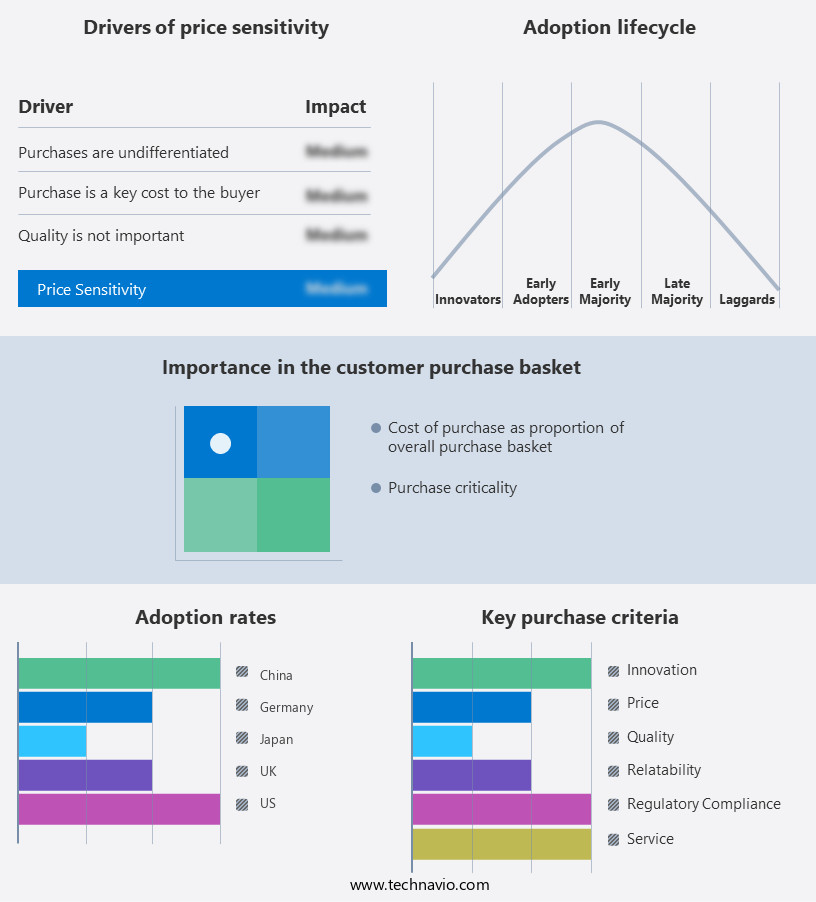
The biopharmaceutical analytical testing services market forecasting report includes the adoption lifecycle of the market, covering from the innovator's stage to the laggard's stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the biopharmaceutical analytical testing services market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape of Biopharmaceutical Analytical Testing Services Industry
Competitive Landscape & Market Insights
Companies are implementing various strategies, such as strategic alliances, biopharmaceutical analytical testing services market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
Almac Group Ltd. - This company specializes in biopharmaceutical analytical testing, providing services for stability testing, raw material analysis, and quality control release testing.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Almac Group Ltd.
- Boston Analytical
- Catalent Inc.
- Charles River Laboratories International Inc.
- Coriolis Pharma Research GmbH
- Curia Global Inc.
- Element Materials Technology
- Eurofins Scientific SE
- ICON plc
- Intertek Group Plc
- Laboratory Corp. of America Holdings
- Merck KGaA
- Pace Analytical Services LLC
- Parexel International Corp.
- SGS SA
- Solvias AG
- STERIS plc
- Thermo Fisher Scientific Inc.
- Vimta Labs Ltd.
- West Pharmaceutical Services Inc.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Biopharmaceutical Analytical Testing Services Market
- In January 2024, Thermo Fisher Scientific, a leading biotech product development and supplier, announced the launch of its new mass spectrometry-based solution, 'Veracity One,' for biopharmaceutical analytical testing services (Thermo Fisher Scientific Press Release, 2024). This innovation offers enhanced accuracy and throughput, addressing the growing demand for advanced analytical testing in the biopharmaceutical industry.
- In March 2024, Merck KGaA and Siemens Healthineers entered into a strategic collaboration to develop an integrated solution for real-time monitoring of biopharmaceutical manufacturing processes (Merck KGaA Press Release, 2024). This partnership combines Merck's expertise in biopharmaceuticals and Siemens Healthineers' advanced technology, aiming to improve efficiency and quality in the manufacturing sector.
- In May 2024, Eurofins Scientific, a global analytical testing services provider, completed the acquisition of BioReliance, a leading contract research organization for biopharmaceutical testing (Eurofins Scientific Press Release, 2024). This acquisition expanded Eurofins' capabilities in the biopharmaceutical testing market, enabling the company to offer a more comprehensive range of services to its clients.
- In February 2025, the U.S. Food and Drug Administration (FDA) approved a significant policy change, allowing for the use of real-time release testing (RTRT) in the biopharmaceutical industry (FDA Press Release, 2025). This approval streamlines the regulatory process, enabling faster market entry for new biopharmaceutical products and reducing the overall time to market.
Dive into Technavio's robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Biopharmaceutical Analytical Testing Services Market insights. See full methodology.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
224 |
|
Base year |
2024 |
|
Historic period |
2019-2023 |
|
Forecast period |
2025-2029 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 15.2% |
|
Market growth 2025-2029 |
USD 5481.3 million |
|
Market structure |
Fragmented |
|
YoY growth 2024-2025(%) |
12.9 |
|
Key countries |
US, China, Japan, UK, Germany, France, Canada, India, South Korea, and Brazil |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
Research Analyst Overview
- In the dynamic and evolving landscape of biopharmaceutical analytical testing services, various techniques and methods play pivotal roles in ensuring pharmaceutical quality control. Microbial limits testing, a critical aspect, employs advanced analytical techniques to detect and quantify microorganisms in drug substances and products. Sample preparation techniques are refined to optimize the accuracy of subsequent tests, such as protein quantification methods and particle analysis techniques. Bioanalytical method validation is essential for ensuring the reliability and robustness of testing methods used in drug substance characterization and pharmaceutical stability testing. Biopharmaceutical quality attributes, including endotoxin detection methods and impurity profiling assays, are rigorously evaluated to maintain the highest standards.
- Pharmaceutical regulatory compliance is achieved through various means, including analytical instrument calibration and method transfer protocols. Mass spectrometry analysis, a versatile and sophisticated technique, is widely used for the identification and quantification of various components in pharmaceutical formulations. Biopharmaceutical testing outsourcing has become increasingly common due to the complexity and cost-effectiveness it offers. Advanced analytical techniques, such as chromatographic separation techniques and spectroscopic identification methods, are employed to meet the growing demands for more precise and accurate testing. Immunogenicity assessment assays are crucial in assessing the potential of a drug to elicit an immune response. Pharmaceutical testing automation streamlines the testing process, enhancing efficiency and reducing human error.
- Biopharmaceutical formulation testing is another essential service, ensuring the stability and efficacy of the final product. Sterility testing protocols and reference standards qualification are integral parts of the testing process, ensuring the safety and quality of the drug substance and final product. The continuous unfolding of market activities and evolving patterns in biopharmaceutical analytical testing services underscores the importance of staying informed and adopting the latest techniques and methodologies.
What are the Key Data Covered in this Biopharmaceutical Analytical Testing Services Market Research and Growth Report?
-
What is the expected growth of the Biopharmaceutical Analytical Testing Services Market between 2025 and 2029?
-
USD 5.48 billion, at a CAGR of 15.2%
-
-
What segmentation does the market report cover?
-
The report segmented by Service (Bioanalytical, Method development and validation, Stability testing, and Others), End-user (Pharma and biotech companies and CROs), Type (Physiochemical analysis, Biological assays, Microbial testing, and Environmental monitoring), and Geography (North America, APAC, Europe, South America, and Middle East and Africa)
-
-
Which regions are analyzed in the report?
-
North America, APAC, Europe, South America, and Middle East and Africa
-
-
What are the key growth drivers and market challenges?
-
Increase in demand for mAbs, Shortage of trained professionals
-
-
Who are the major players in the Biopharmaceutical Analytical Testing Services Market?
-
Key Companies Almac Group Ltd., Boston Analytical, Catalent Inc., Charles River Laboratories International Inc., Coriolis Pharma Research GmbH, Curia Global Inc., Element Materials Technology, Eurofins Scientific SE, ICON plc, Intertek Group Plc, Laboratory Corp. of America Holdings, Merck KGaA, Pace Analytical Services LLC, Parexel International Corp., SGS SA, Solvias AG, STERIS plc, Thermo Fisher Scientific Inc., Vimta Labs Ltd., and West Pharmaceutical Services Inc.
-
Market Research Insights
- The market encompasses a range of specialized techniques and technologies used to ensure the quality and safety of pharmaceutical and biopharmaceutical products. Two significant aspects of this market are method lifecycle management and pharmaceutical testing validation. Method lifecycle management involves the systematic development, validation, and transfer of analytical methods, ensuring their continued suitability throughout a product's life cycle. In contrast, pharmaceutical testing validation ensures the accuracy, reliability, and reproducibility of testing results by validating methods and equipment against established pharmaceutical testing standards.
- For instance, over 70% of pharmaceutical companies outsource analytical testing, while the global market for analytical testing equipment is projected to reach USD 1 1.5 billion by 2025, reflecting the growing importance of these services in maintaining pharmaceutical quality systems and adhering to regulatory requirements.
We can help! Our analysts can customize this biopharmaceutical analytical testing services market research report to meet your requirements.