Blood Warmer Devices Market Size 2026-2030
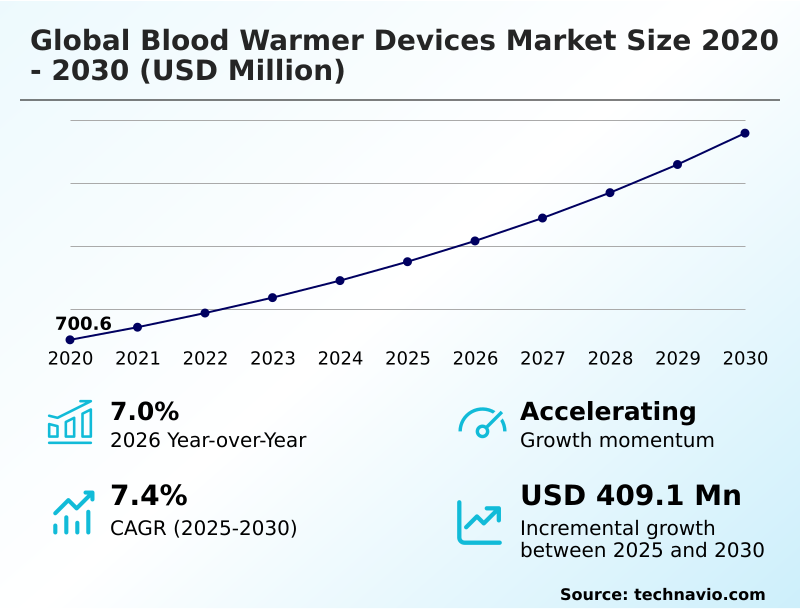
The blood warmer devices market size is valued to increase by USD 409.1 million, at a CAGR of 7.4% from 2025 to 2030. Escalating incidence of trauma cases and complex surgical interventions will drive the blood warmer devices market.
Major Market Trends & Insights
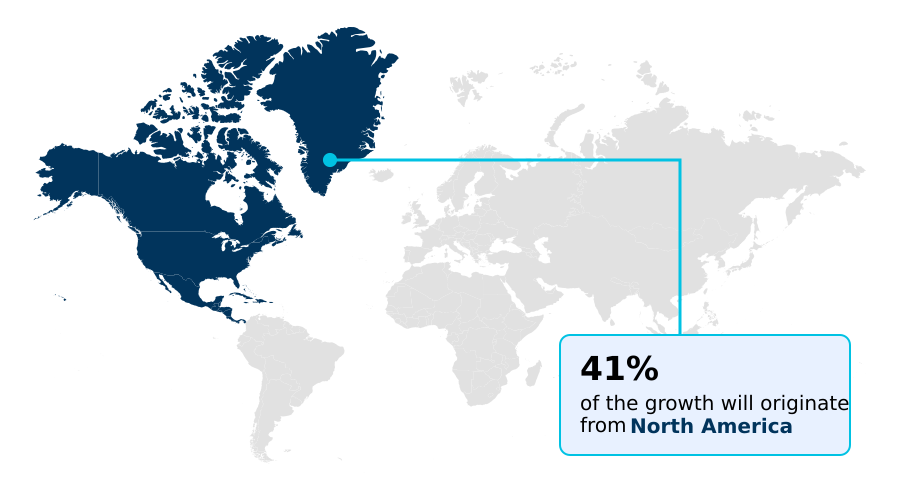
- North America dominated the market and accounted for a 41.4% growth during the forecast period.
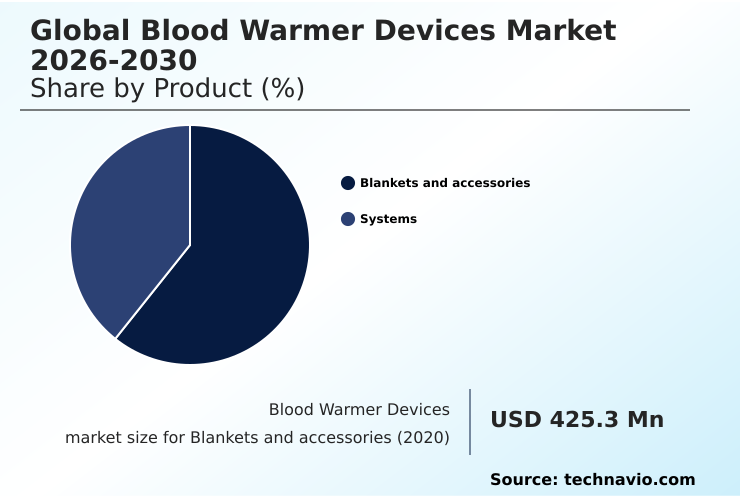
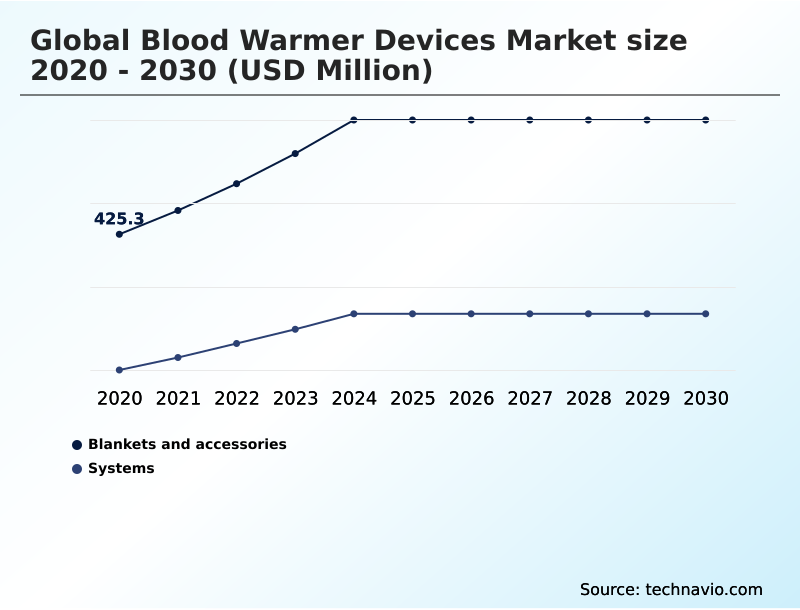
- By Product - Blankets and accessories segment was valued at USD 551.6 million in 2024
- By Application - Surgery segment accounted for the largest market revenue share in 2024
Market Size & Forecast
- Market Opportunities: USD 657.6 million
- Market Future Opportunities: USD 409.1 million
- CAGR from 2025 to 2030 : 7.4%
Market Summary
- The blood warmer devices market is expanding, driven by the increasing volume of complex surgeries and the critical need for normothermia management in trauma care. These devices are essential for preventing perioperative hypothermia by heating blood and intravenous fluids to physiological temperatures, a process crucial for mitigating risks like cardiac distress and coagulopathy.
- A primary trend is the shift toward portable, battery-powered warming solutions, which enables point-of-care warming in pre-hospital settings. For instance, in military medicine, the use of lightweight, ruggedized devices for fluid resuscitation during the 'golden hour' significantly improves patient outcomes. Innovations in dry heat warming and electromagnetic induction are displacing older water-bath systems, reducing contamination risks.
- However, the market faces challenges from stringent regulatory approval cycles and supply chain volatility for specialized components like high-precision thermal sensors and biocompatible polymers. A business scenario involves a hospital system standardizing its thermal regulation protocols across all emergency departments, using devices with wireless data integration to automate documentation and ensure compliance, thereby improving efficiency and patient safety.
What will be the Size of the Blood Warmer Devices Market during the forecast period?
Get Key Insights on Market Forecast (PDF) Request Free Sample
How is the Blood Warmer Devices Market Segmented?
The blood warmer devices industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2026-2030, as well as historical data from 2020-2024 for the following segments.
- Product
- Blankets and accessories
- Systems
- Application
- Surgery
- Emergency and trauma care
- Critical care
- Neonatal care
- End-user
- Hospitals
- Ambulatory surgical centers
- Blood banks
- Specialty clinics
- Geography
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Asia
- Rest of World (ROW)
- North America
By Product Insights
The blankets and accessories segment is estimated to witness significant growth during the forecast period.
The global blood warmer devices market 2026-2030 is segmented by product, application, end-user, and geography. The product segment includes systems and blankets/accessories, with the latter addressing the critical need for perioperative hypothermia prevention.
These accessories, including forced-air warming and carbon fiber resistive heating products, are essential for maintaining normothermia management during prolonged surgeries.
A key market dynamic is the industry's shift toward disposable blankets to mitigate infection risks, a move that directly impacts revenue models for vendors offering patient temperature management solutions. Advanced designs now incorporate lightweight, biocompatible polymers for enhanced usability.
This focus on active fluid warming is crucial, as effective thermal management systems can improve patient outcomes; for instance, proper temperature control has been linked to a reduction in post-operative recovery times for patients.
The evolution of these thermal regulation protocols underscores their foundational role.
The Blankets and accessories segment was valued at USD 551.6 million in 2024 and showed a gradual increase during the forecast period.
Regional Analysis
North America is estimated to contribute 41.4% to the growth of the global market during the forecast period.Technavio’s analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
See How Blood Warmer Devices Market Demand is Rising in North America Request Free Sample
The geographic landscape of the global blood warmer devices market 2026-2030 is led by North America, which accounts for over 41% of the market's incremental growth.
This dominance is due to advanced healthcare infrastructure, high surgical volumes, and stringent patient safety mandates promoting active fluid warming. Europe follows, with a 7.3% CAGR driven by a focus on cost-effectiveness and the adoption of reusable warming components.
Asia is the fastest-growing region, with an 8.3% CAGR, fueled by massive healthcare investments and expanding medical tourism in countries like Thailand and Singapore. The adoption of portable blood warmers and infusion fluid warmers in this region is accelerating.
The implementation of advanced thermal management systems has been shown to enhance heat transfer efficiency, while next-generation solid-state heating elements contribute to improved performance across all regions.
Market Dynamics
Our researchers analyzed the data with 2025 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
- Strategic positioning in the global blood warmer devices market 2026-2030 requires a focus on specialized applications and operational efficiency. Vendors are developing solutions for preventing thermal hemolysis during transfusion, a critical factor in patient safety. The demand for a portable blood warmer for military use continues to drive innovation in rugged, battery-powered designs that are essential for pre-hospital trauma care.
- In surgical settings, the ability to reduce the risk of surgical site infections through consistent normothermia is a key value proposition. Advanced systems now incorporate real-time fluid temperature monitoring, with some achieving a 12% reduction in post-operative recovery times compared to legacy methods.
- For neonatal intensive care, conductive heating for neonatal care offers gentle and precise temperature control for vulnerable infants. Furthermore, the integration of a rapid infuser for trauma care is becoming standard in emergency medicine, enabling swift and effective fluid resuscitation.
- Devices offering automated temperature control for blood products help streamline workflows and minimize human error, ensuring compliance with strict clinical protocols and enhancing overall patient outcomes. This focus on targeted, high-performance solutions addresses the diverse and evolving needs of modern healthcare.
What are the key market drivers leading to the rise in the adoption of Blood Warmer Devices Industry?
- The escalating incidence of trauma cases and complex surgical interventions is a key driver for the market's growth.
- Market growth is significantly driven by the escalating incidence of trauma cases and complex surgeries, which necessitates effective hypothermia-induced coagulopathy prevention.
- The use of automated fluid warming systems in level-one trauma centers has been demonstrated to reduce post-operative recovery times by 12%, a compelling driver for hospital investment. Technological advancements, particularly in portable devices, are expanding market reach.
- For example, next-generation solid-state heating elements offer heat transfer efficiencies 30% higher than previous models, enabling faster warm-up times and longer battery life.
- Furthermore, the strengthening of clinical guidelines mandating active fluid warming, especially in neonatal and pediatric surgeries, creates a sustained demand. This convergence of clinical necessity, technological innovation, and regulatory support ensures the continued adoption of advanced blood warming solutions.
What are the market trends shaping the Blood Warmer Devices Industry?
- The integration of digital intelligence with precise thermal systems is a significant upcoming market trend. This evolution transforms blood warmers into sophisticated, data-generating instruments for enhanced clinical oversight.
- A primary trend in the global blood warmer devices market 2026-2030 is the integration of digital intelligence, transforming these devices into smart, connected instruments. Modern systems provide real-time thermal monitoring to prevent thermal injury and ensure fluids are maintained at a precise thirty-seven degrees Celsius.
- The proliferation of portable and battery-powered warming solutions, some offering over six hours of continuous operation, addresses the critical need for point-of-care warming in emergency and military medicine. This shift is enabled by breakthroughs in induction heating and energy-efficient resistive films.
- The adoption of eco-friendly manufacturing practices, including the use of recyclable polymers, is also gaining traction, reflecting a broader industry commitment to sustainability and reducing the environmental footprint of medical hardware.
What challenges does the Blood Warmer Devices Industry face during its growth?
- Stringent regulatory compliance requirements and the burden of certification standards present a key challenge affecting industry growth.
- The global blood warmer devices market 2026-2030 faces challenges from stringent and complex regulatory landscapes, which prolong approval cycles and increase development costs. For instance, updates to medical device regulations can force redesigns to incorporate features like data logging, adding significant R&D overhead.
- Supply chain instability poses another significant hurdle, with disruptions in specialized components like semiconductor chips and medical-grade polymers leading to production bottlenecks. A major labor strike recently caused a nearly 25% increase in procurement costs for essential raw materials, impacting manufacturers' margins.
- Additionally, economic constraints and infrastructure gaps in emerging economies limit the adoption of advanced warming systems, particularly those requiring stable electrical grids and costly disposable sets, creating disparities in patient care.
Exclusive Technavio Analysis on Customer Landscape
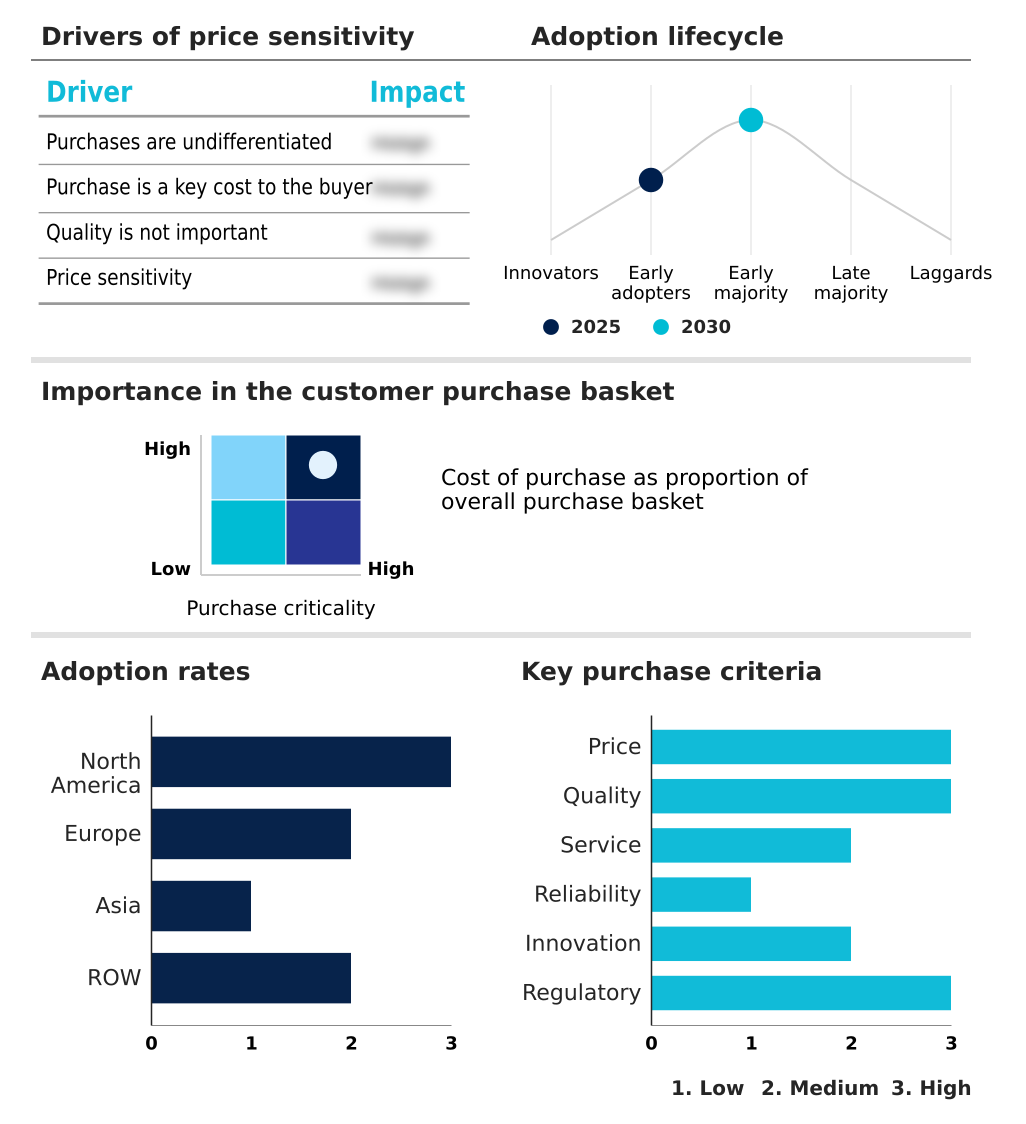
The blood warmer devices market forecasting report includes the adoption lifecycle of the market, covering from the innovator’s stage to the laggard’s stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the blood warmer devices market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape of Blood Warmer Devices Industry
Competitive Landscape
Companies are implementing various strategies, such as strategic alliances, blood warmer devices market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
3M Co. - Offerings include fluid warming systems and integrated patient warming solutions designed for advanced thermal management in diverse clinical settings.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- 3M Co.
- Barkey GmbH and Co. KG
- Becton Dickinson and Co.
- Belmont Medical Tech.
- BIEGLER GmbH
- EMIT Corp.
- Estill Medical Tech Inc.
- Gentherm Inc.
- ICU Medical Inc.
- LIFE WARMER
- MEQU
- Narang Medical Ltd.
- SARSTEDT AG and Co. KG
- Sino Medical Tech Co. Ltd.
- Smisson Cartledge Bio LLC
- Stryker Corp.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Blood warmer devices market
- In August 2024, Quality In Flow, also known as QinFlow, released the Warrior AC Station, a next-generation warming solution tailored for intensive care units and operating rooms.
- In February 2025, a prominent research consortium based in North America announced the successful implementation of a machine learning algorithm designed to predict blood flow rate and adjust heating output to maintain an exact temperature.
- In March 2025, a leading American medical device manufacturer received regulatory approval for a high-speed fluid warmer that utilizes electromagnetic induction for precise temperature control.
- In April 2025, a comprehensive study by a prominent North American healthcare research collective revealed that integrating automated fluid warming systems in level-one trauma centers resulted in a twelve percent reduction in post-operative recovery times.
- In May 2025, Stryker Corp. participated in the Bank of America Healthcare Conference, where leadership reported significant procedural growth in the trauma and extremities business segments, indicating rising demand for ancillary technologies like blood warming instruments.
Dive into Technavio’s robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Blood Warmer Devices Market insights. See full methodology.
| Market Scope | |
|---|---|
| Page number | 296 |
| Base year | 2025 |
| Historic period | 2020-2024 |
| Forecast period | 2026-2030 |
| Growth momentum & CAGR | Accelerate at a CAGR of 7.4% |
| Market growth 2026-2030 | USD 409.1 million |
| Market structure | Fragmented |
| YoY growth 2025-2026(%) | 7.0% |
| Key countries | US, Canada, Mexico, Germany, UK, France, Italy, Spain, The Netherlands, China, Japan, India, South Korea, Indonesia, Thailand, Brazil, Saudi Arabia, UAE, South Africa, Turkey, Israel, Argentina and Colombia |
| Competitive landscape | Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
Research Analyst Overview
- The global blood warmer devices market 2026-2030 is advancing beyond basic temperature regulation, with a pronounced shift toward integrated systems that enhance clinical decision-making. The core technology is evolving from simple dry heat warming to sophisticated electromagnetic induction and carbon-nanotube technology, delivering superior performance in fluid resuscitation.
- A key boardroom consideration is the adoption of devices that ensure compliance with strengthening clinical guidelines for normothermia management. For instance, new solid-state heating elements achieve heat transfer efficiencies 30% higher than previous models, a quantifiable metric that translates to faster patient stabilization and reduced energy consumption.
- The market is also characterized by a focus on preventing complications like thermal hemolysis and perioperative hypothermia through high-flow warming solutions and micro-flow warming technology. The development of biocompatible polymers for disposable warming sets further addresses infection control priorities.
- This convergence of advanced patient temperature management and data-driven oversight positions blood warmers as a critical component of modern surgical and emergency care.
What are the Key Data Covered in this Blood Warmer Devices Market Research and Growth Report?
-
What is the expected growth of the Blood Warmer Devices Market between 2026 and 2030?
-
USD 409.1 million, at a CAGR of 7.4%
-
-
What segmentation does the market report cover?
-
The report is segmented by Product (Blankets and accessories, and Systems), Application (Surgery, Emergency and trauma care, Critical care, and Neonatal care), End-user (Hospitals, Ambulatory surgical centers, Blood banks, and Specialty clinics) and Geography (North America, Europe, Asia, Rest of World (ROW))
-
-
Which regions are analyzed in the report?
-
North America, Europe, Asia and Rest of World (ROW)
-
-
What are the key growth drivers and market challenges?
-
Escalating incidence of trauma cases and complex surgical interventions, Stringent regulatory compliance and burden of certification standards
-
-
Who are the major players in the Blood Warmer Devices Market?
-
3M Co., Barkey GmbH and Co. KG, Becton Dickinson and Co., Belmont Medical Tech., BIEGLER GmbH, EMIT Corp., Estill Medical Tech Inc., Gentherm Inc., ICU Medical Inc., LIFE WARMER, MEQU, Narang Medical Ltd., SARSTEDT AG and Co. KG, Sino Medical Tech Co. Ltd., Smisson Cartledge Bio LLC and Stryker Corp.
-
Market Research Insights
- The blood warmer devices market is defined by a push toward greater efficiency and safety, with technological advancements yielding measurable clinical and operational benefits. The adoption of automated fluid warming systems has been shown to reduce post-operative recovery times by 12% in level-one trauma centers, directly impacting hospital throughput.
- Similarly, new solid-state heating elements utilizing advanced carbon-nanotube technology offer heat transfer efficiencies 30% higher than previous models, enabling faster warm-up times and extended battery life for portable units. This focus on real-time thermal monitoring and hemodynamic stability is critical.
- Innovations in micro-flow warming technology have proven to lower the incidence of cold stress in preterm infants, showcasing the move toward specialized, high-precision applications. These developments in patient temperature management reflect a market prioritizing data-driven outcomes and enhanced thermal regulation protocols across all points of care.
We can help! Our analysts can customize this blood warmer devices market research report to meet your requirements.