Carpal Tunnel Release Systems Market Size 2024-2028
The carpal tunnel release systems market size is forecast to increase by USD 531.3 million at a CAGR of 6.5% between 2023 and 2028.
- The market is experiencing significant growth, driven primarily by the increasing prevalence of Carpal Tunnel Syndrome (CTS) worldwide. CTS is a common condition that causes pain, numbness, and tingling sensations in the hand and wrist, often leading to decreased productivity and quality of life. As a result, there is a rising demand for effective treatment options, with endoscopic carpal tunnel release ries gaining popularity due to their minimally invasive nature and faster recovery time. However, market growth is not without challenges. Stringent regulations and quality standards set by regulatory bodies are necessitating significant investments in research and development to ensure the safety and efficacy of carpal tunnel release systems.
- These regulations aim to mitigate risks associated with surgical procedures and improve patient outcomes. Companies seeking to capitalize on market opportunities must navigate these regulatory hurdles while also addressing evolving patient expectations and competition from alternative treatment modalities. Effective strategic planning and operational agility will be key to success in this dynamic market.
What will be the Size of the Carpal Tunnel Release Systems Market during the forecast period?
- The market in the US is experiencing significant growth due to the increasing prevalence of nerve injuries, particularly those affecting the hand and wrist. This market is driven by advancements in healthcare technology, which enable more precise and minimally invasive surgical procedures for carpal tunnel release. The focus on preventing surgical complications and promoting faster post-operative recovery is also fueling market expansion. Additionally, the shift towards personalized healthcare and virtual healthcare services is creating new opportunities for carpal tunnel treatment. The rise of regenerative approaches and telehealth services is making carpal tunnel treatment more accessible and affordable for a wider patient base.
- However, data security and protection remain critical concerns in this market, as medical devices used in carpal tunnel release procedures often involve sensitive patient information. Medical device innovation continues to play a key role in addressing these challenges and improving overall patient outcomes. Carpal tunnel syndrome causes hand and wrist pain, numbness, and weakness, which can significantly impact daily activities. The market for carpal tunnel release systems is expected to continue growing as the need for effective and efficient treatment solutions increases. The market's direction is towards more precise and minimally invasive procedures, with a focus on reducing recovery time and improving patient comfort.
How is this Carpal Tunnel Release Systems Industry segmented?
The carpal tunnel release systems industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2024-2028, as well as historical data from 2018-2022 for the following segments.
- Product
- Open carpal tunnel release system
- Endoscopic carpal tunnel release system
- Geography
- North America
- US
- Canada
- Europe
- France
- Germany
- UK
- Asia
- Rest of World (ROW)
- North America
By Product Insights
The open carpal tunnel release system segment is estimated to witness significant growth during the forecast period.
Carpal tunnel release systems are medical devices used to alleviate symptoms of carpal tunnel syndrome, a condition characterized by pain, numbness, and weakness in the hand and wrist. This condition, which can affect pregnant women, individuals with diabetes, rheumatoid arthritis, high blood pressure, or those experiencing fluid retention, results from pressure on the median nerve due to swelling or compression of the wrist's tiny bones. Open carpal tunnel release procedures involve making an incision in the wrist to move the ligaments out of the way, relieving pressure on the median nerve. In some cases, the procedure may include cutting certain bones at the base of the hand and removing tissues from inside the arm.
These procedures are typically performed under local anesthesia and take approximately 30 minutes to an hour to complete. Patients may experience immediate relief from symptoms, but full recovery can take several weeks. Healthcare infrastructure plays a crucial role in ensuring the availability and affordability of these procedures. Advanced surgical systems and power tools have streamlined the process, making it more cost-effective for healthcare services. However, risks such as infection, bleeding, nerve aggravation, and scarring remain concerns. Skilled professionals must perform these procedures to optimize patient outcomes and minimize complications. Carpal tunnel release systems have significant benefits for individuals dealing with excessive strain on their wrists, which can be exacerbated by vibrations, pressure, and weakness due to conditions like osteoporosis and sarcopenia.
As healthcare expenditure continues to rise, cost-effective solutions like these will remain essential in addressing the needs of an aging population.
Get a glance at the market report of share of various segments Request Free Sample
The Open carpal tunnel release system segment was valued at USD 723.90 million in 2018 and showed a gradual increase during the forecast period.
Regional Analysis
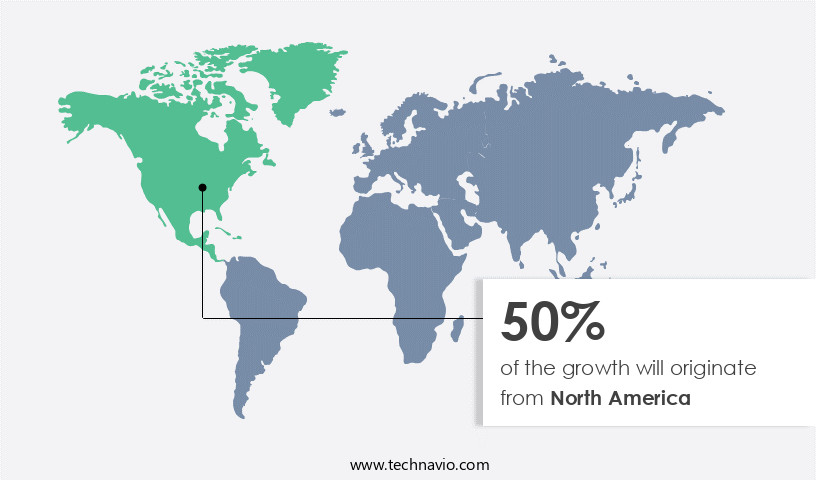
North America is estimated to contribute 50% to the growth of the global market during the forecast period.Technavio’s analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
For more insights on the market size of various regions, Request Free Sample
The market in the US is driven by the high prevalence of carpal tunnel syndrome among pregnant women and the aging population. With approximately 3-6% of adults in the US experiencing this condition in the past decade, according to a study by the Louisiana State University Health Sciences Centre, the demand for effective treatment solutions is on the rise. This market is also influenced by the increasing healthcare expenditure due to conditions like diabetes, rheumatoid arthritis, osteoporosis, and high blood pressure, which are known to aggravate carpal tunnel syndrome. Advanced surgical systems, such as power tools and minimally invasive techniques, offer benefits like reduced scarring, quicker recovery, and improved patient outcomes.
However, concerns around infection, bleeding, and nerve aggravation during ry remain. Skilled professionals use local anesthesia to minimize discomfort during open ry, which involves cutting the ligaments in the wrist to release pressure on the median nerve. The cost-effectiveness of these medical devices, along with the availability of affordable options, makes them an attractive choice for healthcare services. The market growth is further fueled by the development of new products, such as advanced surgical technology and products designed for specific patient needs, like those with sarcopenia or hand vibration issues. Despite these advancements, challenges like excessive strain from repetitive motions and fluid retention remain, which could impact the long-term success of the market.
In summary, the market in the US is driven by the increasing prevalence of carpal tunnel syndrome, advanced surgical systems, and the aging population. The market faces challenges like infection, bleeding, and nerve aggravation, but the development of new products and cost-effectiveness make it an attractive investment for healthcare services.
Market Dynamics
Our researchers analyzed the data with 2023 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
What are the key market drivers leading to the rise in the adoption of Carpal Tunnel Release Systems Industry?
- Increasing prevalence of carpal tunnel syndrome (CTS) is the key driver of the market.
- The market is experiencing significant growth due to the increasing number of cases of Carpal Tunnel Syndrome (CTS). A report published in February 2022 revealed that the shift to remote work during the pandemic led to a notable rise in repetitive stress injuries, with nearly 30% of remote workers experiencing symptoms related to CTS. The report underscores the relevance of this condition in today's work environments. According to research, CTS affects approximately 1 to 3 people per 1,000 annually in the US, with a prevalence rate of around 50 per 1,000.
- These figures are consistent with findings in other countries, highlighting the global impact of this condition. The market growth is further driven by advancements in technology and the increasing demand for minimally invasive surgical procedures. Despite the challenges posed by the pandemic, the market is expected to continue its expansion in the forecast period.
What are the market trends shaping the Carpal Tunnel Release Systems Industry?
- Rise in demand for endoscopic carpal tunnel release ries is the upcoming market trend.
- The market is experiencing notable growth due to the rising preference for minimally invasive endoscopic carpal tunnel release (ECTR) ries. Advanced surgical techniques have driven this trend, as ECTR offers several advantages over traditional open ries. These benefits include reduced postoperative pain, minimal scarring, and faster recovery times. The increasing awareness of these advantages among patients has led to a growing number of individuals opting for ECTR as a treatment option for carpal tunnel syndrome. Furthermore, the prevalence of conditions causing carpal tunnel syndrome, such as repetitive strain injuries from prolonged computer use, is also contributing to the market's expansion.
- The market is expected to witness substantial growth, with endoscopic techniques playing a pivotal role in improving patient outcomes and satisfaction.
What challenges does the Carpal Tunnel Release Systems Industry face during its growth?
- Stringent regulations is a key challenge affecting the industry growth.
- Medical device manufacturers, including those producing carpal tunnel release systems, must comply with regulations set forth by government agencies such as the Food and Drug Administration (FDA) and the Center for Medicare Services (CMS) within the U.S. Department of Health and Human Services. These agencies oversee various aspects of medical devices, from design and testing to manufacturing, labeling, promoting, and marketing. Adhering to these regulations is a complex and costly process. In the U.S., the FDA's Center for Devices and Radiological Health (CDRH) is responsible for regulating the development, testing, manufacturing, labeling, and marketing of medical devices.
- These regulations vary by country, necessitating manufacturers to adapt to different regulatory frameworks for global distribution. Ensuring compliance with these regulations is crucial for manufacturers to deliver safe and effective products to consumers.
Exclusive Customer Landscape
The carpal tunnel release systems market forecasting report includes the adoption lifecycle of the market, covering from the innovator’s stage to the laggard’s stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the carpal tunnel release systems market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape
Key Companies & Market Insights
Companies are implementing various strategies, such as strategic alliances, carpal tunnel release systems market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
A.M. Surgical Inc. - The company specializes in carpal tunnel release systems, including the A M Surgical Endoscopic Carpal Tunnel Release System. This innovative solution employs a minimally invasive technique to alleviate pressure on the median nerve via precise instrumentation, enhancing patient recovery and reducing post-procedure complications. By utilizing advanced technology, the system ensures accurate and efficient relief for those suffering from carpal tunnel syndrome.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- A.M. Surgical Inc.
- Arthrex Inc.
- Conmed Corp.
- Innomed Inc.
- Integra LifeSciences Holdings Corp.
- LB Medical LLC
- Medical Designs LLC
- MicroAire Surgical Instruments LLC
- Nordson Corp.
- S2S Surgical LLC
- Smith and Nephew plc
- Sonex Health Inc.
- Stryker Corp.
- Trice Medical
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Research Analyst Overview
The carpal tunnel release system market encompasses a range of medical devices designed to alleviate pressure on the median nerve in the wrist. This condition, characterized by symptoms such as pain, weakness, and numbness, can result from various factors including fluid retention, excessive strain, and health conditions like diabetes and rheumatoid arthritis. The prevalence of carpal tunnel syndrome is influenced by several factors. The aging population, with its increased susceptibility to conditions like osteoporosis and sarcopenia, is a significant contributor. Additionally, the use of power tools and repetitive motions in industries and daily life can lead to excessive strain on the wrist.
The healthcare infrastructure and availability of advanced surgical systems play a crucial role in the market. Open surgical methods, which involve making an incision to release the ligament pressing on the median nerve, have been the traditional approach. However, the development of less invasive, cost-effective alternatives has gained popularity. These alternatives include endoscopic and ultrasound-guided procedures, which offer benefits such as reduced scarring, quicker recovery times, and lower healthcare costs. Despite these advancements, challenges persist. Infection, bleeding, and nerve aggravation are potential risks associated with carpal tunnel release procedures. Furthermore, the affordability and availability of these medical devices, especially in developing regions, remain concerns.
Patient outcomes are a key focus in the carpal tunnel release system market. Factors such as the skill of the professional performing the procedure, the use of local anesthesia, and the individual patient's response to treatment all influence the success of the intervention. The market dynamics are influenced by various factors. The increasing prevalence of conditions leading to carpal tunnel syndrome, the development of more cost-effective and less invasive surgical technologies, and the ongoing need to balance cost and effectiveness are all significant trends. Furthermore, the impact of pandemics on healthcare services and healthcare expenditure may also influence the market.
In , the carpal tunnel release system market represents a critical area of development in healthcare technology. With a focus on patient outcomes, cost-effectiveness, and minimally invasive approaches, this market continues to evolve to meet the needs of an aging population and the demands of various industries.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
157 |
|
Base year |
2023 |
|
Historic period |
2018-2022 |
|
Forecast period |
2024-2028 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 6.5% |
|
Market growth 2024-2028 |
USD 531.3 million |
|
Market structure |
Concentrated |
|
YoY growth 2023-2024(%) |
6.0 |
|
Key countries |
US, Germany, Canada, UK, and France |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
What are the Key Data Covered in this Carpal Tunnel Release Systems Market Research and Growth Report?
- CAGR of the Carpal Tunnel Release Systems industry during the forecast period
- Detailed information on factors that will drive the growth and forecasting between 2024 and 2028
- Precise estimation of the size of the market and its contribution of the industry in focus to the parent market
- Accurate predictions about upcoming growth and trends and changes in consumer behaviour
- Growth of the market across North America, Europe, Asia, and Rest of World (ROW)
- Thorough analysis of the market’s competitive landscape and detailed information about companies
- Comprehensive analysis of factors that will challenge the carpal tunnel release systems market growth of industry companies
We can help! Our analysts can customize this carpal tunnel release systems market research report to meet your requirements.