Intravenous (IV) Fluid Bags Market Size 2025-2029
The intravenous (iv) fluid bags market size is forecast to increase by USD 940.5 million, at a CAGR of 6.3% between 2024 and 2029.
- The market is experiencing significant growth due to the increasing prevalence of chronic diseases, leading to an increased demand for IV fluids in healthcare settings. Chronic diseases such as diabetes, cancer, and cardiovascular diseases are on the rise, resulting in a higher number of patients requiring IV fluid therapy. Moreover, innovations and advancements in material technology have led to the development of superior IV fluid bags with improved functionality and enhanced patient safety. However, the market faces challenges related to the availability of raw materials and high pricing. The scarcity of raw materials, such as PVC and DEHP, has resulted in price fluctuations and supply chain disruptions.
- Companies in the IV Fluid Bags Market must navigate these challenges by exploring alternative raw material sources and implementing cost-effective production methods to maintain competitiveness and meet the growing demand for IV fluids. To capitalize on market opportunities and mitigate challenges effectively, strategic business decisions and operational planning should focus on supply chain optimization, research and development of alternative raw materials, and cost management.
What will be the Size of the Intravenous (IV) Fluid Bags Market during the forecast period?
The IV fluid bags market is a dynamic and evolving sector that caters to various industries, including healthcare, pharmaceuticals, and veterinary medicine. Low-density polyethylene (LDPE) and ethylene vinyl acetate (EVA) are commonly used materials in the production of these bags due to their inherent properties, such as flexibility, transparency, and resistance to chemicals. In healthcare settings, IV fluid bags are essential for nutrient delivery, electrolyte balance, and critical care. Infection control is a significant concern, leading to stringent regulations on particle count, leak testing, and regulatory compliance. Infusion pumps, such as syringe pumps and infusion sets, facilitate the controlled administration of fluids for bolus injection, intermittent infusion, and continuous infusion.
Pharmaceutical manufacturing relies on IV fluid bags for bulk packaging and drug delivery. Quality control and volume control are crucial factors in ensuring therapeutic efficacy. In veterinary medicine, IV fluid bags are used for emergency medicine, blood volume expansion, and patient safety. The market for IV fluid bags is continually unfolding, with ongoing research and development in materials, manufacturing processes, and application areas. Fluid management systems, including administration sets, drip chambers, and storage conditions, are essential components of the IV fluid bag market. The supply chain management of these products is also a critical aspect of the market's dynamics.
In summary, the IV fluid bags market is a dynamic and evolving sector that caters to various industries, with a focus on ensuring patient safety, therapeutic efficacy, and regulatory compliance. The use of LDPE, EVA, and other materials continues to evolve, along with advancements in infusion technology, storage conditions, and supply chain management.
How is this Intravenous (IV) Fluid Bags Industry segmented?
The intravenous (iv) fluid bags industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2025-2029, as well as historical data from 2019-2023 for the following segments.
- Product
- PVC material
- Non-PVC material
- End-user
- Hospitals
- Home healthcare
- Others
- Product Type
- Single-chamber IV bags
- Multi-chamber IV bags
- Geography
- North America
- US
- Canada
- Europe
- France
- Germany
- Italy
- Spain
- UK
- APAC
- China
- Japan
- South America
- Brazil
- Rest of World (ROW)
- North America
By Product Insights
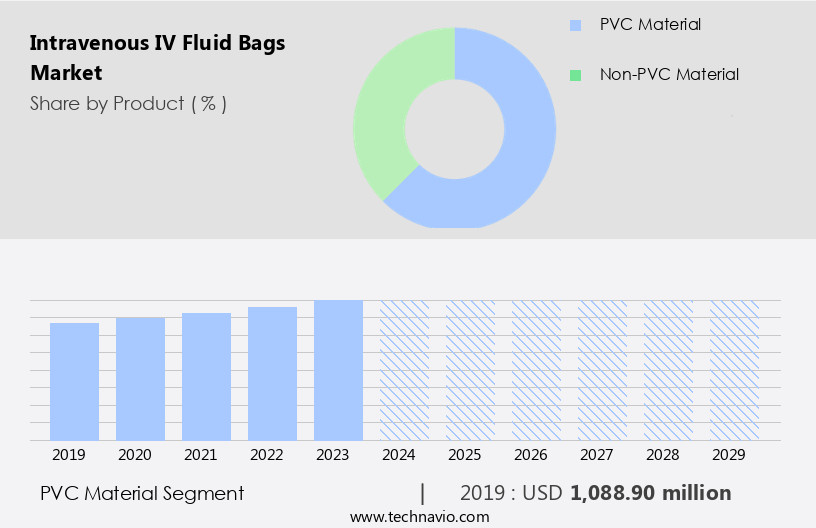
The pvc material segment is estimated to witness significant growth during the forecast period.
Intravenous (IV) fluid bags, particularly those made of polyvinyl chloride (PVC), dominate the global market due to their affordability, versatility, and ease of production. PVC's strength, lightweight, and non-toxic properties make it an ideal material for medical applications. Transparency is another significant advantage, enabling healthcare professionals to accurately monitor fluid levels and administer treatments. PVC bags are compatible with a broad spectrum of pharmaceutical substances, catering to various medical needs. Infection control is paramount in this industry, leading to stringent regulations on particle count and leak testing. Contract manufacturing, unit-dose packaging, and syringe pumps are essential solutions for maintaining patient safety and regulatory compliance.
Infusion pumps, intermittent infusion, and continuous infusion are critical components of IV fluid delivery systems. Intravenous fluids, including those for critical care, electrolyte balance, blood volume expansion, and nutrient delivery, are essential in emergency medicine and veterinary medicine. Home healthcare and pharmaceutical manufacturing also rely on bulk packaging and fluid management for therapeutic efficacy. High-density and low-density polyethylene, as well as ethylene vinyl acetate, are alternative materials used in the production of IV fluid bags. Regardless of the material, quality control, volume control, and supply chain management are essential to ensure the reliability and consistency of these vital medical products.
The PVC material segment was valued at USD 1088.90 million in 2019 and showed a gradual increase during the forecast period.
Regional Analysis
North America is estimated to contribute 32% to the growth of the global market during the forecast period.Technavio's analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
The market in North America is experiencing significant growth, driven by several key factors. With a well-established healthcare system and stringent regulatory environment, the region is a significant contributor to the global market. The aging population and the rising prevalence of chronic diseases are primary reasons for the increasing demand for IV fluids and related products in North America. According to World Bank data, the geriatric population in the US, which was 17% of the total population as of 2023, requires frequent hospitalizations and long-term care, leading to a higher need for IV fluids and solutions. Infection control is a critical concern in the IV fluid market, with particle count being a significant factor.
Manufacturers are focusing on advanced technologies, such as contract manufacturing, unit-dose packaging, and leak testing, to ensure patient safety and regulatory compliance. Infusion pumps, including syringe pumps and continuous infusion systems, are essential components of IV fluid administration, with emerging trends favoring the use of smart pumps and electronic health records for improved fluid management. IV fluids are used in various applications, including critical care, emergency medicine, intermittent infusion, blood volume expansion, and nutrient delivery. Materials used in the production of IV fluid bags include polyvinyl chloride, high-density polyethylene, and low-density polyethylene, among others. Proper storage conditions and leak testing are crucial to maintain the therapeutic efficacy and safety of IV fluids.
The market for IV fluids and related products is diverse, encompassing various applications, materials, and technologies, and is subject to continuous innovation and improvement.
Market Dynamics
Our researchers analyzed the data with 2024 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
What are the key market drivers leading to the rise in the adoption of Intravenous (IV) Fluid Bags Industry?
- The escalating incidence of chronic diseases serves as the primary market catalyst.
- The global market for intravenous (IV) fluid bags is experiencing significant growth due to the increasing prevalence of chronic diseases. Chronic conditions, such as cardiovascular disease, diabetes, and cancer, are on the rise and require long-term treatment, making intravenous therapy an essential part of patient care. According to the World Health Organization (WHO), chronic diseases accounted for approximately 74% of global mortality in 2023. In response to this growing demand, there is a focus on developing effective and reliable drug delivery methods. IV fluid bags are a popular option for delivering drugs, nutrients, and fluids directly into the bloodstream.
- These bags are typically made of high-density polyethylene and include components such as administration sets, volume control, drip chambers, and quality control features. Effective supply chain management is crucial to ensure the timely delivery of these essential medical supplies. In addition, the increasing trend of home healthcare is driving the demand for IV fluid bags in this sector. Overall, the market for IV fluid bags is expected to continue growing as the need for effective and reliable drug delivery methods increases.
What are the market trends shaping the Intravenous (IV) Fluid Bags Industry?
- The current market trend reflects a significant focus on innovations and advancements in material technology. This sector is experiencing substantial growth and development.
- Intravenous (IV) fluid bags have been a crucial component of nutrient delivery in pharmaceutical manufacturing for several decades. Initially, polyvinyl chloride (PVC) was the primary material used due to its flexibility and cost-effectiveness. However, concerns regarding the environmental impact and potential health risks associated with PVC's plasticizers have led to the exploration of alternative materials. Polyolefins, such as low-density polyethylene (LDPE), and ethylene vinyl acetate (EVA), are gaining popularity in the production of IV fluid bags. These materials offer advantages like improved fluid management and therapeutic efficacy while reducing environmental concerns and health risks. The shift towards these materials signifies the adaptability of intravenous fluid bag manufacturers to the evolving needs of healthcare providers and patients.
- The focus on developing more sustainable and safe alternatives to PVC is a significant trend in the intravenous fluid bag market. As material technology advances, manufacturers continue to innovate and provide solutions that cater to the ever-changing landscape of healthcare. By prioritizing the use of eco-friendly materials and enhancing the overall quality and performance of IV fluid bags, manufacturers are ensuring the delivery of safe and effective nutrient solutions for various therapeutic applications.
What challenges does the Intravenous (IV) Fluid Bags Industry face during its growth?
- The industry's expansion is hindered by the complex issue of limited raw material availability and elevated pricing.
- The global IV fluid bags market faces several challenges, primarily due to the volatile availability of raw materials, such as polyvinyl chloride, which is extensively used in their production. Geopolitical tensions and natural disasters can lead to supply chain disruptions, resulting in increased production times, delays, and higher costs. Furthermore, the cost of raw materials, including plastics derived from petroleum, significantly impacts market prices. Infection control is a critical concern in the IV fluid bags industry, with particle count and leak testing being essential quality checks.
- Contract manufacturing and unit-dose packaging are popular trends, ensuring sterility and reducing the risk of contamination. In critical care settings, maintaining electrolyte balance is crucial, and IV solutions cater to various therapeutic requirements, including bolus injections. Despite these challenges, the market continues to evolve, with innovation in technology and manufacturing processes driving advancements in infection control, cost-effectiveness, and patient safety.
Exclusive Customer Landscape
The intravenous (iv) fluid bags market forecasting report includes the adoption lifecycle of the market, covering from the innovator's stage to the laggard's stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the intravenous (iv) fluid bags market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape
Key Companies & Market Insights
Companies are implementing various strategies, such as strategic alliances, intravenous (iv) fluid bags market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
Alfa Laboratories S.A.L. - This company specializes in providing intravenous (IV) fluid bags, including Sodium Chloride Injection USP, for medical use. The IV bag is equipped with antimicrobial agents to ensure patient safety and is intended for single-dose administration only. Adherence to this usage ensures the maintenance of sterility and effectiveness. Our offerings prioritize quality and adhere to regulatory standards, enhancing search engine exposure for healthcare professionals seeking reliable IV solutions.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Alfa Laboratories S.A.L.
- B.Braun SE
- Baxter International Inc.
- Becton Dickinson and Co.
- Fosmo Med
- Fresenius SE and Co. KGaA
- ICU Medical Inc.
- Kraton Corp.
- Macopharma SA
- Medicopack
- Otsuka Holdings Co. Ltd.
- Pharmaceutical Solutions Industry
- Polifarma Ilac San. ve Tic. A.S.
- PolyCine GmbH
- RENOLIT SE
- Sartorius AG
- SIPPEX
- Technoflex
- The Metrix Co.
- Vioser SA
- Wellpharma
- West Pharmaceutical Services Inc.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Intravenous (IV) Fluid Bags Market
- In February 2023, B. Braun Melsungen AG, a leading medical device manufacturer, introduced the Plasmanet Flex Infusion System, an advanced IV fluid warming and administration system. This innovation allows for customized temperature control and real-time monitoring of infusion parameters, enhancing patient safety and comfort (B. Braun Press Release, 2023).
- In May 2024, Fresenius Kabi, a global healthcare company, entered into a strategic partnership with Grifols, a leading producer of plasma-derived medicines, to expand its IV fluid portfolio. This collaboration aims to leverage Grifols' expertise in plasma-derived IV solutions and Fresenius Kabi's distribution network, strengthening their market position (Fresenius Kabi Press Release, 2024).
- In August 2024, Hospira, a Pfizer Inc. Subsidiary, received FDA approval for its new line of sterile IV fluid bags, featuring advanced antimicrobial technology. These bags are designed to reduce the risk of bacterial contamination during infusion, addressing a significant concern in the healthcare industry (FDA Press Release, 2024).
- In November 2025, Terumo Corporation, a Japanese medical device manufacturer, announced a major investment in its production facility in the United States to increase IV fluid bag production capacity by 50%. This expansion is in response to the growing demand for IV fluids, particularly in the wake of the COVID-19 pandemic (Terumo Press Release, 2025).
Research Analyst Overview
- The IV fluid bags market is characterized by stringent regulations and a focus on ensuring product safety and quality. Sterile filtration and cleanroom manufacturing are essential in producing pharmaceutical-grade fluids. Drug development involves rigorous validation protocols to ensure drug compatibility and prevent product recalls. Gamma irradiation and e-beam sterilization are common methods for ensuring aseptic processing and maintaining the sterility of aqueous solutions. Adherence to USP standards and European Pharmacopeia guidelines is crucial for regulatory approvals. Just-in-time delivery and inventory management are essential for minimizing environmental impact. ISO standards and electronic data capture are integral to product traceability, ensuring accountability and transparency throughout the supply chain.
- Clinical trials require careful consideration of drug compatibility and adverse events, with batch records meticulously maintained to ensure safety and efficacy. RFID technology facilitates efficient tracking and monitoring, enhancing overall supply chain management. The market for IV fluid bags continues to evolve, with a focus on innovation and sustainability.
Dive into Technavio's robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Intravenous (IV) Fluid Bags Market insights. See full methodology.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
212 |
|
Base year |
2024 |
|
Historic period |
2019-2023 |
|
Forecast period |
2025-2029 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 6.3% |
|
Market growth 2025-2029 |
USD 940.5 million |
|
Market structure |
Fragmented |
|
YoY growth 2024-2025(%) |
5.7 |
|
Key countries |
US, China, Germany, Japan, France, UK, Canada, Italy, Brazil, and Spain |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
What are the Key Data Covered in this Intravenous (IV) Fluid Bags Market Research and Growth Report?
- CAGR of the Intravenous (IV) Fluid Bags industry during the forecast period
- Detailed information on factors that will drive the growth and forecasting between 2025 and 2029
- Precise estimation of the size of the market and its contribution of the industry in focus to the parent market
- Accurate predictions about upcoming growth and trends and changes in consumer behaviour
- Growth of the market across North America, Europe, Asia, and Rest of World (ROW)
- Thorough analysis of the market's competitive landscape and detailed information about companies
- Comprehensive analysis of factors that will challenge the intravenous (iv) fluid bags market growth of industry companies
We can help! Our analysts can customize this intravenous (iv) fluid bags market research report to meet your requirements.