Pharmacovigilance And Drug Safety Software Market Size 2026-2030
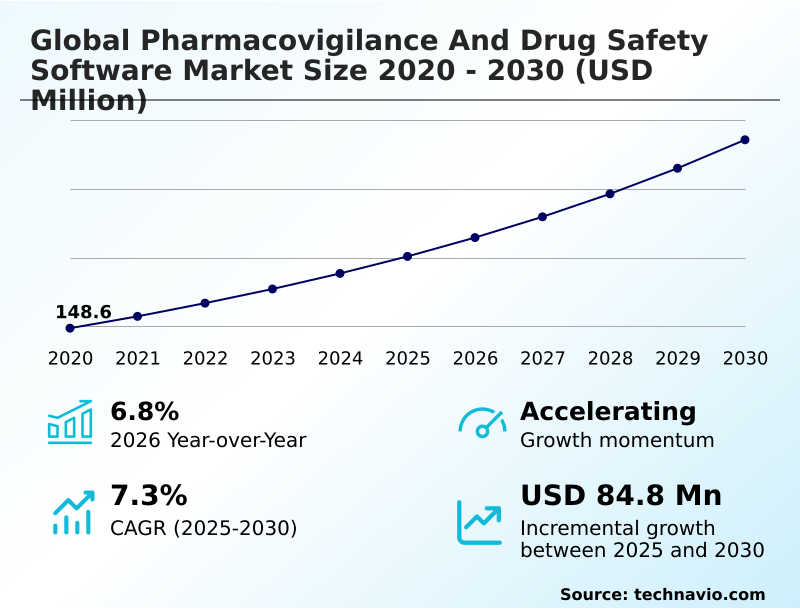
The pharmacovigilance and drug safety software market size is valued to increase by USD 84.8 million, at a CAGR of 7.3% from 2025 to 2030. Rising incidence rates of adverse drug events will drive the pharmacovigilance and drug safety software market.
Major Market Trends & Insights
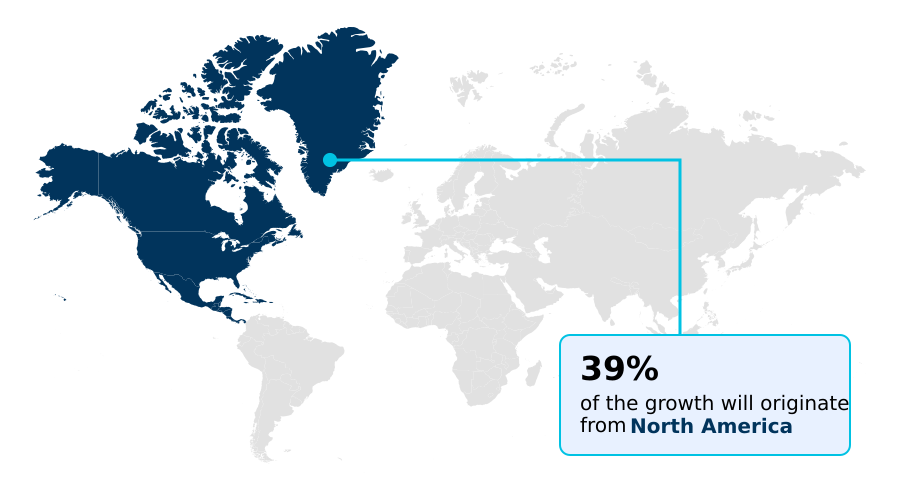
- North America dominated the market and accounted for a 39.2% growth during the forecast period.
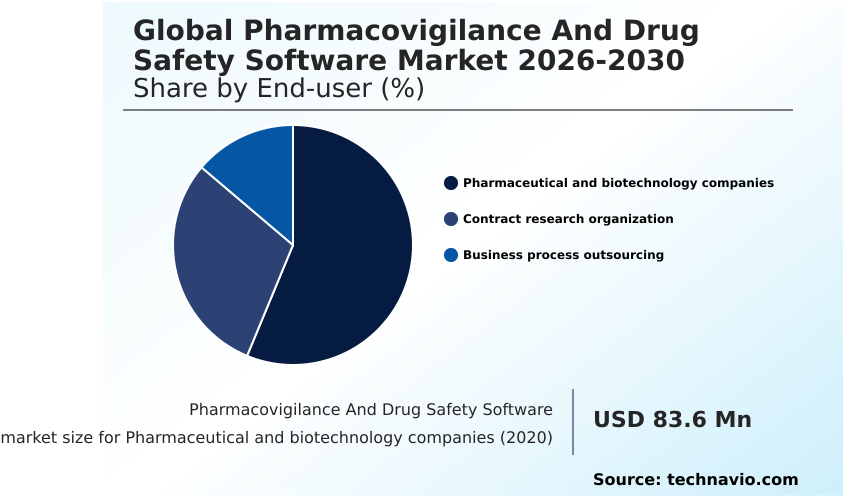
- By End-user - Pharmaceutical and biotechnology companies segment was valued at USD 107.1 million in 2024
- By Deployment - Cloud-based segment accounted for the largest market revenue share in 2024
Market Size & Forecast
- Market Opportunities: USD 137 million
- Market Future Opportunities: USD 84.8 million
- CAGR from 2025 to 2030 : 7.3%
Market Summary
- The pharmacovigilance and drug safety software market is fundamentally shaped by the need to ensure patient safety amid increasingly complex global regulations. Key drivers include the rising volume of adverse drug events and the stringent compliance mandates from health authorities worldwide, which necessitate sophisticated systems for data collection, analysis, and reporting.
- A primary trend is the rapid adoption of artificial intelligence and machine learning to automate routine tasks, such as case processing and signal detection, which enhances efficiency and accuracy. Cloud-based solutions are also becoming standard, offering scalability and reducing the infrastructure burden for companies of all sizes.
- For instance, a mid-sized biotechnology firm launching its first drug globally can leverage a cloud-based software-as-a-service platform to manage safety reporting across different regions without a large upfront capital investment, ensuring timely compliance.
- However, the market faces challenges related to the high cost of implementation, data security concerns in a cloud environment, and the difficulty of integrating new software with existing legacy IT systems. Addressing these issues is crucial for vendors aiming to provide effective and accessible solutions that help life sciences companies navigate the intricate landscape of drug safety and regulatory adherence.
What will be the Size of the Pharmacovigilance And Drug Safety Software Market during the forecast period?
Get Key Insights on Market Forecast (PDF) Request Free Sample
How is the Pharmacovigilance And Drug Safety Software Market Segmented?
The pharmacovigilance and drug safety software industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2026-2030, as well as historical data from 2020-2024 for the following segments.
- End-user
- Pharmaceutical and biotechnology companies
- Contract research organization
- Business process outsourcing
- Deployment
- Cloud-based
- On-premises
- Application
- Post-marketing surveillance
- Clinical trials
- Regulatory compliance
- Geography
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Asia
- Rest of World (ROW)
- North America
By End-user Insights
The pharmaceutical and biotechnology companies segment is estimated to witness significant growth during the forecast period.
The pharmaceutical and biotechnology sector's demand is driven by stringent regulatory requirements and the need for robust benefit-risk assessment.
These organizations leverage dedicated software for pre-market and post-market safety studies, employing causality assessment tools and safety signal management to identify unrecognized adverse events.
With high R&D spending, managing the pharmacovigilance system master file and preparing aggregate reporting tools like periodic safety update reports are critical.
The integration of electronic health record integration is a key focus, with modern platforms improving the efficiency of PV literature screening by over 40%.
Companies also utilize pharmacovigilance consulting to refine their pharmacovigilance business strategy and ensure compliance for products like medical device vigilance and cosmetovigilance software, while managing pharmacovigilance SOPs and complex tasks such as E2B R3 conversion and MedDRA coding automation.
The Pharmaceutical and biotechnology companies segment was valued at USD 107.1 million in 2024 and showed a gradual increase during the forecast period.
Regional Analysis
North America is estimated to contribute 39.2% to the growth of the global market during the forecast period.Technavio’s analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
See How Pharmacovigilance And Drug Safety Software Market Demand is Rising in North America Request Free Sample
The global landscape is led by North America, which accounts for over 39% of market opportunities, driven by its stringent regulatory environment that mandates advanced signal detection analytics and E2B(R3) compliance.
Europe remains a significant region, with a strong focus on comprehensive risk management planning and pharmacovigilance risk assessment. The fastest growth is seen in Asia, where pharmacovigilance outsourcing to CRO pharmacovigilance platforms is common.
These service providers leverage systems with multi-tenant architecture to manage global client portfolios efficiently. The adoption of case processing automation and automated case intake in this region has improved operational efficiency by up to 35%.
A key focus across all mature regions is clinical trial safety management and the implementation of a rigorous signal validation process to support benefit-risk management and facilitate ongoing pharmacovigilance training.
Market Dynamics
Our researchers analyzed the data with 2025 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
- Strategic decision-making for pharmacovigilance systems requires a thorough evaluation of multiple factors. A primary consideration is the cloud versus on-premise PV debate, where the drug safety software TCO is often a deciding factor. For emerging biotech pharmacovigilance platforms, scalable solutions are critical, whereas established firms may prioritize a robust global safety database selection process.
- The integration of AI-powered signal detection and machine learning in signal detection is becoming a standard expectation, with systems leveraging NLP for adverse events to process unstructured data. Ensuring compliance requires solutions for E2B R3 compliance solutions and automated MedDRA coding tools. For organizations conducting clinical studies, managing data from EHR data integration software is essential.
- The functionality must also extend to medical device vigilance software and support for PSUR management software features. Key operational aspects include selecting the right pharmacovigilance audit trail software, planning for data migration in pharmacovigilance, and engaging PV software validation services.
- Ultimately, the choice of automated case processing tools and a comprehensive risk management plan software is vital for ensuring patient data security in PV and maintaining regulatory adherence. Systems that streamline these processes are shown to accelerate reporting cycles significantly compared to legacy methods.
What are the key market drivers leading to the rise in the adoption of Pharmacovigilance And Drug Safety Software Industry?
- The rising incidence of adverse drug events is a key driver fueling the demand for advanced pharmacovigilance and drug safety software.
- Increasingly stringent global regulations and the rising incidence of adverse drug reactions are primary drivers compelling investment in advanced drug safety monitoring solutions.
- Health authorities demand strict GVP modules compliance and flawless regulatory submission management, making a comprehensive pharmacovigilance compliance software essential.
- Modern adverse event reporting system platforms provide an end-to-end safety platform for the entire drug development safety lifecycle, from IND safety reporting to post-marketing surveillance.
- The adoption of specialized adverse drug reaction software facilitates efficient e-reporting for pharmacovigilance, reducing critical errors in drug safety audits.
- This need is amplified by the continuous approval of new molecules, which requires robust patient safety monitoring software to manage a higher volume of data and ensure timely reporting, improving compliance rates to over 98% in many cases.
What are the market trends shaping the Pharmacovigilance And Drug Safety Software Industry?
- The integration of artificial intelligence is an emerging trend in pharmacovigilance. This shift is reshaping data management and analytical capabilities across the industry.
- The market is undergoing significant transformation, driven by the adoption of a cloud-based SaaS platform model and the pervasive influence of AI in pharmacovigilance. This trend enables the deployment of sophisticated biopharmaceutical safety solutions and integrated safety suite offerings with greater scalability.
- Utilizing machine learning automation and natural language processing, modern systems can analyze unstructured data from sources like social media listening for PV, improving the accuracy of AI-based safety case processing by up to 30%. The focus on real-world evidence integration and real-world data analytics is also paramount, with advanced pharmacovigilance analytics providing deeper insights.
- Cloud pharmacovigilance solutions not only enhance life sciences compliance but also reduce infrastructure maintenance costs by over 40% compared to traditional on-premises deployments.
What challenges does the Pharmacovigilance And Drug Safety Software Industry face during its growth?
- The high total cost of ownership, including installation and maintenance, presents a significant challenge to market growth, particularly for smaller organizations.
- Key market challenges center on operational and security concerns. The complexity of maintaining data integrity and security within a global safety database or any pharmacovigilance database remains a top priority, especially with healthcare data breaches being 35% more frequent than in other regulated industries.
- Integrating new validated software systems with legacy pharmacovigilance IT infrastructure often presents significant hurdles, leading to data silos and hampering PV data management. Furthermore, the cost and complexity associated with drug safety services, such as maintaining safety data exchange agreements and ensuring PV audit readiness, are substantial.
- The management of a regulatory intelligence platform, vigilance platform technology, and related drug safety call center solutions also requires significant investment, while ensuring PSMF maintenance adds to the operational burden for many organizations.
Exclusive Technavio Analysis on Customer Landscape
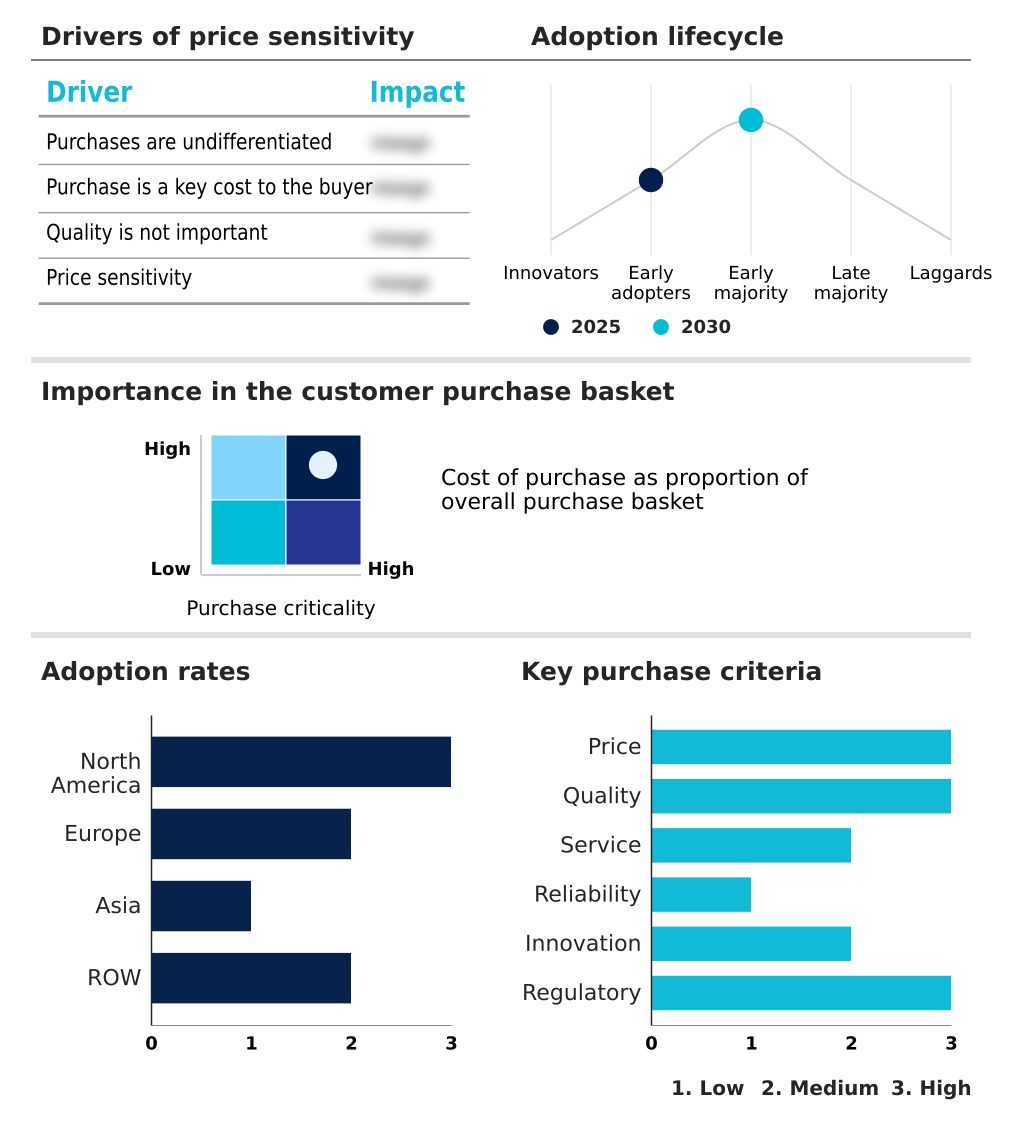
The pharmacovigilance and drug safety software market forecasting report includes the adoption lifecycle of the market, covering from the innovator’s stage to the laggard’s stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the pharmacovigilance and drug safety software market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape of Pharmacovigilance And Drug Safety Software Industry
Competitive Landscape
Companies are implementing various strategies, such as strategic alliances, pharmacovigilance and drug safety software market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
AB Cube SARL - Offerings center on unified, cloud-native platforms that provide end-to-end safety case management, automated regulatory reporting, and advanced analytics to ensure global compliance for life sciences firms.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- AB Cube SARL
- Accenture PLC
- ArisGlobal LLC
- BaseCon AS
- Clarivate PLC
- Cognizant Technology Solution
- Ennov SAS
- EXTEDO GmbH
- Honeywell International Inc.
- Indegene Pvt. Ltd.
- IQVIA Holdings Inc.
- Max Application Srl
- Oracle Corp.
- Pegasystems Inc.
- Sarjen Systems Pvt. Ltd.
- TriNetX LLC
- United BioSource LLC
- Veeva Systems Inc.
- Wipro Ltd.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Pharmacovigilance and drug safety software market
- In September 2024, ReguSure AI announced the launch of its SignalSphere platform, which uses machine learning automation to analyze real-world evidence from disparate sources, enhancing proactive safety signal management for pharmaceutical clients (Source: Company Press Release).
- In November 2024, VigilanceTech Solutions acquired DataSafe Analytics for approximately $350 million, integrating its advanced pharmacovigilance analytics with VigilanceTech's established cloud-based SaaS platform to create a comprehensive end-to-end safety platform (Source: The Wall Street Journal).
- In February 2025, OmniCloud Pharma and HealthSys EHR entered a strategic partnership to embed OmniCloud's automated case intake module directly within the HealthSys electronic health record system, simplifying adverse event reporting for healthcare providers by over 50% (Source: Reuters).
- In April 2025, BioSafety Platforms Inc. secured $100 million in Series C funding to expand its multi-tenant architecture, specifically targeting contract research organizations and enhancing its clinical trial safety management capabilities for decentralized trials (Source: Bloomberg).
Dive into Technavio’s robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Pharmacovigilance And Drug Safety Software Market insights. See full methodology.
| Market Scope | |
|---|---|
| Page number | 294 |
| Base year | 2025 |
| Historic period | 2020-2024 |
| Forecast period | 2026-2030 |
| Growth momentum & CAGR | Accelerate at a CAGR of 7.3% |
| Market growth 2026-2030 | USD 84.8 million |
| Market structure | Fragmented |
| YoY growth 2025-2026(%) | 6.8% |
| Key countries | US, Canada, Mexico, Germany, UK, France, Italy, Spain, The Netherlands, China, Japan, India, South Korea, Singapore, Indonesia, Brazil, Australia, Saudi Arabia, UAE, Argentina, South Africa, Colombia and Egypt |
| Competitive landscape | Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
Research Analyst Overview
- The pharmacovigilance and drug safety software market is driven by the absolute necessity for life sciences companies to maintain regulatory compliance and ensure patient safety. The adoption of a modern adverse event reporting system is no longer optional but a core operational requirement.
- Boardroom decisions increasingly center on investing in a validated software system that incorporates a global safety database and robust pharmacovigilance database functionalities. Key technologies shaping the landscape include real-world evidence integration, natural language processing, and machine learning automation for advanced pharmacovigilance analytics.
- The implementation of case processing automation can reduce manual data entry efforts by as much as 60%, allowing teams to focus on high-value activities like benefit-risk assessment, causality assessment tools, and safety signal management.
- Solutions must support the entire lifecycle, from clinical trial safety management to post-marketing surveillance, including drug safety audits, MedDRA coding automation, and the generation of periodic safety update reports and other aggregate reporting tools. Ensuring data integrity and security, managing a pharmacovigilance system master file, and enabling seamless electronic health record integration are pivotal for a compliant, end-to-end safety platform.
What are the Key Data Covered in this Pharmacovigilance And Drug Safety Software Market Research and Growth Report?
-
What is the expected growth of the Pharmacovigilance And Drug Safety Software Market between 2026 and 2030?
-
USD 84.8 million, at a CAGR of 7.3%
-
-
What segmentation does the market report cover?
-
The report is segmented by End-user (Pharmaceutical and biotechnology companies, Contract research organization, and Business process outsourcing), Deployment (Cloud-based, and On-premises), Application (Post-marketing surveillance, Clinical trials, and Regulatory compliance) and Geography (North America, Europe, Asia, Rest of World (ROW))
-
-
Which regions are analyzed in the report?
-
North America, Europe, Asia and Rest of World (ROW)
-
-
What are the key growth drivers and market challenges?
-
Rising incidence rates of adverse drug events, High cost of ownership, installation, and maintenance
-
-
Who are the major players in the Pharmacovigilance And Drug Safety Software Market?
-
AB Cube SARL, Accenture PLC, ArisGlobal LLC, BaseCon AS, Clarivate PLC, Cognizant Technology Solution, Ennov SAS, EXTEDO GmbH, Honeywell International Inc., Indegene Pvt. Ltd., IQVIA Holdings Inc., Max Application Srl, Oracle Corp., Pegasystems Inc., Sarjen Systems Pvt. Ltd., TriNetX LLC, United BioSource LLC, Veeva Systems Inc. and Wipro Ltd.
-
Market Research Insights
- Market dynamics are increasingly shaped by a strategic shift towards pharmacovigilance workflow optimization. Organizations are adopting end-to-end safety platforms and integrated safety suites to streamline drug safety services and enhance their PV quality management system. The adoption of AI in pharmacovigilance is pivotal, with some systems improving case processing efficiency by over 30%.
- This move is supported by a robust pharmacovigilance IT infrastructure and a focus on drug development safety. As companies engage in pharmacovigilance outsourcing to CRO pharmacovigilance platforms, demand for scalable drug safety monitoring solutions grows.
- Adherence to GVP modules compliance is non-negotiable, and specialized adverse drug reaction software that facilitates e-reporting for pharmacovigilance can reduce submission errors by up to 90% compared to manual methods, reinforcing the importance of modern vigilance platform technology.
We can help! Our analysts can customize this pharmacovigilance and drug safety software market research report to meet your requirements.