Vaccine Research Market Size 2024-2028
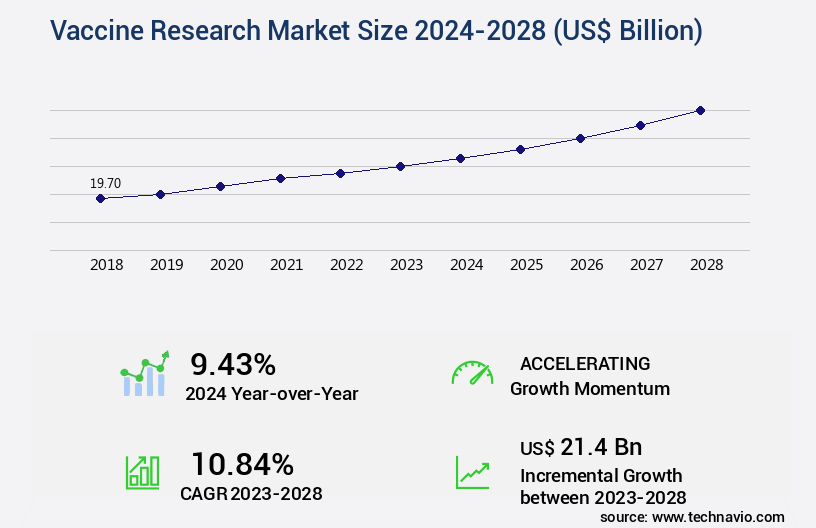
The vaccine research market size is forecast to increase by USD 21.4 billion, at a CAGR of 10.84% between 2023 and 2028.
Major Market Trends & Insights
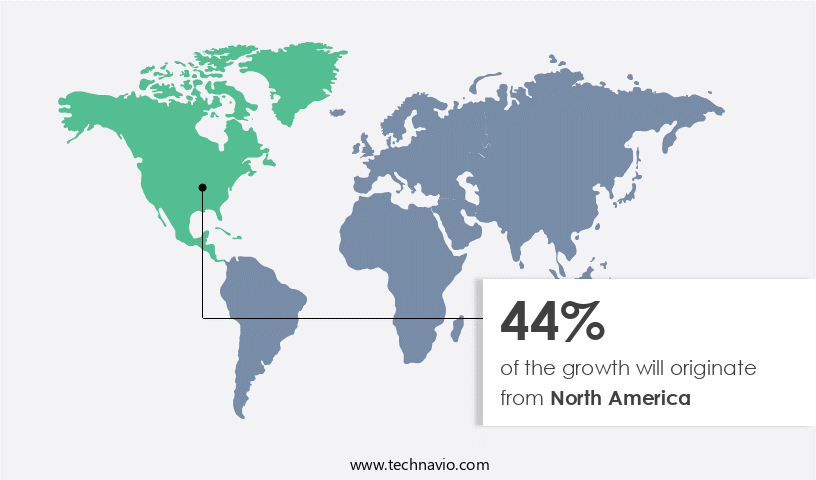
- North America dominated the market and accounted for a 44% growth during the forecast period.
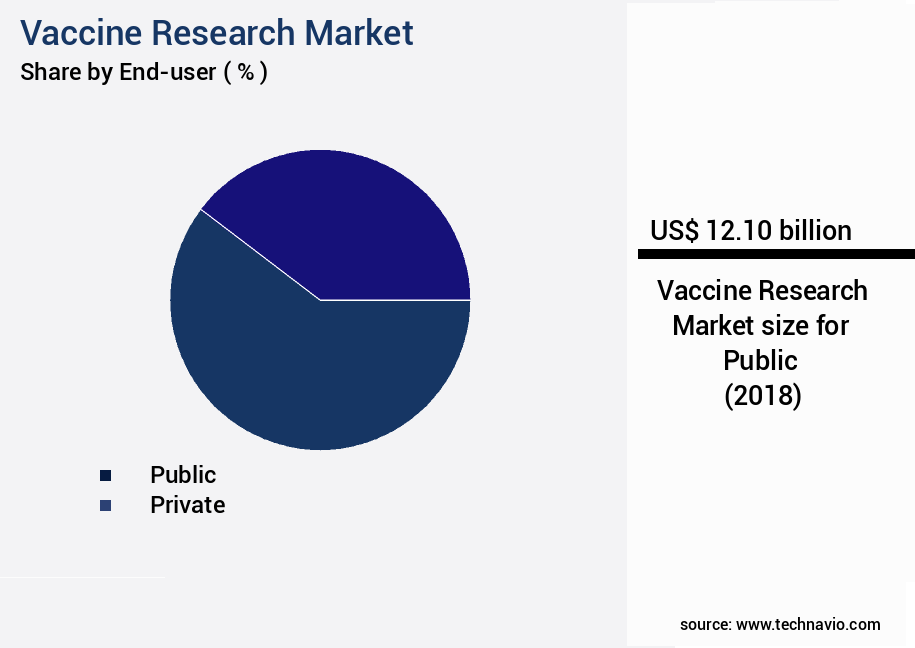
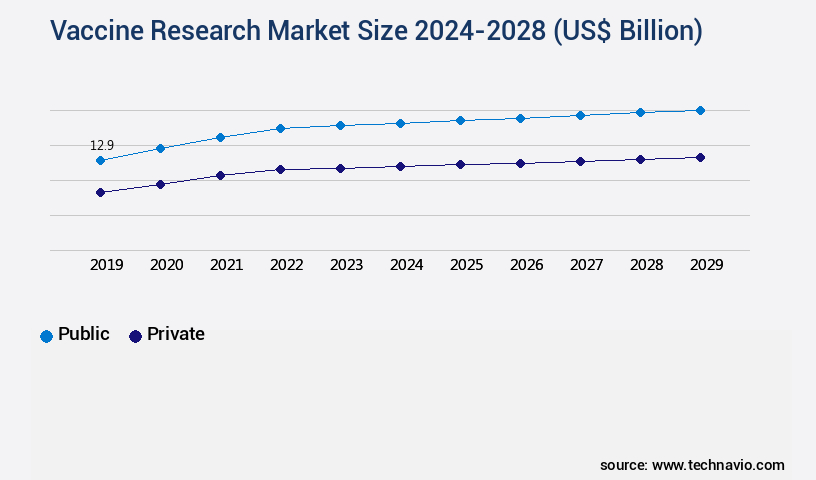
- By the End-user - Public segment was valued at USD 12.10 billion in 2022
- By the Age Group - Pediatric segment accounted for the largest market revenue share in 2022
Market Size & Forecast
- Market Opportunities: USD 170.05 billion
- Market Future Opportunities: USD 21.40 billion
- CAGR : 10.84%
- North America: Largest market in 2022
Market Summary
- The market is a dynamic and innovative sector, driven by advancements in biotechnology and pharmaceuticals. According to recent reports, the global vaccine market size was valued at over USD50 billion in 2020, with a significant increase in investments in research and development. This growth can be attributed to the ongoing efforts to address various infectious diseases, including those that pose a significant public health threat. Notably, the adoption of artificial intelligence (AI) and machine learning (ML) technologies has accelerated vaccine research, enabling faster identification of potential vaccines and reducing the time-to-market. For instance, AI algorithms can analyze vast amounts of data from clinical trials, predicting potential side effects and optimizing dosages.
- Furthermore, ML models can identify patterns in disease outbreaks, helping researchers to focus their efforts on developing vaccines for the most pressing health concerns. Despite these advancements, the high cost of vaccine research, development, and manufacturing remains a significant challenge. However, collaborations between public and private entities, as well as government funding, are helping to mitigate these costs and ensure that vaccines remain accessible to those who need them most. Overall, the market continues to evolve, driven by the need to address new and emerging health threats and improve global health outcomes.
What will be the Size of the Vaccine Research Market during the forecast period?
Explore market size, adoption trends, and growth potential for vaccine research market Request Free Sample
- The market encompasses the development, manufacturing, and testing of various vaccine types, including live attenuated, RNA, recombinant, subunit, conjugate, peptide, inactivated, and virus-like particle vaccines. Two significant milestones in this dynamic industry are the completion of phase 3 trials and the initiation of process validation. For instance, in phase 3 trials, cohort studies involving thousands of participants assess vaccine efficacy and safety. Simultaneously, process validation ensures the consistency and reliability of manufacturing processes for vaccine production. Live attenuated vaccines, such as those based on the measles virus, have historically accounted for a substantial market share.
- However, the advent of RNA vaccines, like those developed for COVID-19, has introduced innovative technologies and expanded the vaccine development pipeline. In 2020, over 200 vaccines were in various stages of clinical development, with approximately 50 in phase 3 trials. This underscores the continuous evolution and growth of the market, driven by ongoing research in adjuvants, immune profiling, immune modulation, and safety assessments.
How is this Vaccine Research Industry segmented?
The vaccine research industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD billion" for the period 2024-2028, as well as historical data from 2018-2022 for the following segments.
- End-user
- Public
- Private
- Age Group
- Pediatric
- Adult
- Technology
- Recombinant Vaccines
- mRNA Vaccines
- Subunit Vaccines
- Live-Attenuated Vaccines
- Inactivated Vaccines
- Viral Vector Vaccines
- Conjugate Vaccines
- Toxoid Vaccines
- Adjuvants
- Disease Type
- Infectious Diseases
- Cancer
- Autoimmune Diseases
- Allergies
- Neurological Disorders
- Research Phase
- Preclinical Research
- Clinical Trials (Phase I, Phase II, Phase III)
- Discovery & Development
- End-User
- Pharmaceutical & Biotechnology Companies
- Academic & Research Institutions
- Contract Research Organizations (CROs)
- Government Agencies
- Geography
- North America
- US
- Canada
- Europe
- France
- Germany
- Italy
- UK
- Middle East and Africa
- Egypt
- KSA
- Oman
- UAE
- APAC
- China
- India
- Japan
- South America
- Argentina
- Brazil
- Rest of World (ROW)
- North America
By End-user Insights
The public segment is estimated to witness significant growth during the forecast period.
The market is driven by the ongoing global health crisis and the need for effective vaccines against various diseases. According to recent reports, the vaccine distribution market is projected to expand by 18.3% in 2021, as governments and private organizations continue to invest heavily in vaccine research and development. MRNA technology, a key area of focus in vaccine research, is expected to grow by 21.5% in the same year. This technology, which uses small molecules to deliver genetic material into cells to produce a specific protein, has been instrumental in the development of several COVID-19 vaccines.
Viral inactivation methods, drug delivery systems, immune response prediction, epitope mapping, and vaccine efficacy assessments are other critical areas of vaccine research that are witnessing substantial growth. Clinical trial design, b cell response, antibody response, and immunogenicity assays are essential components of the vaccine development process. Viral vector platforms, cold chain logistics, immuno-oncology vaccines, protein subunit vaccines, and humoral immunity are some of the other areas of vaccine research that are gaining traction. Manufacturing processes, disease modeling, and quality control testing are also crucial aspects of the market. The market for Therapeutic Vaccines, which are designed to treat existing diseases rather than prevent them, is projected to grow by 15.6% in 2021.
Serological testing, safety monitoring, personalized vaccines, pathogen surveillance, regulatory compliance, and t cell response are some of the key trends in this segment. Adjuvant systems, which enhance the body's immune response to antigens, and antigen design are other areas of vaccine research that are witnessing significant growth. The market is a dynamic and evolving landscape, with ongoing research and development efforts focused on addressing the challenges of vaccine stability, immunological response, dosage optimization, and regulatory compliance.
The Public segment was valued at USD 12.10 billion in 2018 and showed a gradual increase during the forecast period.
Regional Analysis
North America is estimated to contribute 44% to the growth of the global market during the forecast period.Technavio's analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
See How Vaccine Research Market Demand is Rising in North America Request Free Sample
The market in North America is marked by substantial growth, driven by the high prevalence of both infectious and non-infectious diseases. In the US alone, diseases like influenza, hepatitis A, hepatitis B, HIV, measles, malaria, tuberculosis, and cancer continue to pose significant health concerns. The prevalence of these diseases has been increasing at a notable rate, particularly in developed countries. For instance, influenza cases in the US surge every year during November and remain high until January-February. Although the incidence of infectious diseases like mumps, measles, and rubella (MMR) has decreased, they have not been entirely eradicated. The market in North America is expected to grow steadily in the coming years due to the increasing burden of diseases and the continuous efforts to develop effective vaccines.
According to recent studies, the market is projected to expand by approximately 12% in the next five years. Furthermore, the market is anticipated to grow at a similar pace in the following five-year period. This growth can be attributed to the increasing research and development activities, technological advancements, and growing awareness about the importance of vaccines in disease prevention. Additionally, the rising geriatric population and the increasing prevalence of chronic diseases are expected to fuel the market growth. Overall, the market in North America is a dynamic and evolving landscape, driven by the ongoing efforts to combat various diseases and improve public health.
Market Dynamics
Our researchers analyzed the data with 2023 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
Advancements and Innovations in the US the market: Enhancing Efficacy, Compliance, and Performance The US the market is witnessing significant advancements, driven by the pursuit of novel vaccine delivery mechanisms, improved vaccine efficacy against variants, and long-term immune response duration. Next-generation vaccine platforms, such as mRNA and viral vector technologies, are revolutionizing the industry, enabling faster development times and enhanced response. Adjuvant combinations and multivalent vaccine formulations are also gaining traction, boosting the immune system's response and broadening protection against various diseases. Predictive modeling of immune response and personalized vaccine approaches are further pushing the boundaries of innovation, ensuring better compliance and individualized healthcare solutions. Optimization of vaccine manufacturing processes and immunogenicity assessment methods contribute to increased efficiency and safety, reducing downtime and ensuring regulatory compliance. The safety and tolerability profile of vaccines is a critical focus, with ongoing research into vaccine antigen stability and different immune response dynamics. Vaccine distribution strategies, cold chain management, and effectiveness monitoring are essential aspects of the market, ensuring that vaccines reach those in need and maintain their potency. Comparative immunogenicity studies and regulatory approval processes are undergoing digital transformations, streamlining the development and approval of new vaccines.
In conclusion, the US the market is at the forefront of innovation, delivering performance improvements, efficiency gains, and enhanced compliance through advancements in technology, adjuvants, and manufacturing processes. These developments will continue to shape the industry and address the evolving needs of public health and global immunization initiatives.
What are the key market drivers leading to the rise in the adoption of Vaccine Research Industry?
- The high prevalence of infectious diseases serves as the primary market driver, as the ongoing need to develop effective treatments and preventative measures persists.
- The market plays a crucial role in addressing the persistent threat posed by infectious diseases to global health and economic development. This dynamic industry is dedicated to the research, development, and production of vaccines to prevent various infectious diseases. Vaccines are essential tools in protecting populations from the spread of diseases and reducing their impact on public health and economic growth. In developing countries, the market holds significant importance due to the high burden of infectious diseases and the need for improved healthcare infrastructure. In these regions, vaccines can help prevent the devastating consequences of diseases, particularly in the pediatric population.
- For instance, vaccines for measles, polio, and diphtheria have led to a substantial reduction in mortality rates and morbidity among children in these regions. In developed countries, the market continues to be an essential component of healthcare systems, focusing on the development of vaccines for emerging and re-emerging diseases, such as HIV and influenza. The ongoing research and development efforts aim to improve vaccine efficacy, safety, and affordability, ensuring that populations remain protected against infectious diseases and their economic burden is minimized. The market is characterized by continuous innovation and evolution, driven by advancements in technology and scientific research.
- For example, the development of mRNA technology for vaccine production has led to the rapid creation of vaccines for diseases like COVID-19. This technology has revolutionized the vaccine industry by enabling the production of vaccines in record time and at scale. The market's impact is far-reaching, extending beyond healthcare to various sectors, including education, travel, and employment. The availability of vaccines allows for the safe resumption of activities in these sectors, contributing to economic growth and development. According to data from the World Health Organization (WHO), vaccines prevent an estimated 2-3 million deaths annually. This underscores the importance of the market in addressing the ongoing threat posed by infectious diseases and contributing to global health and economic development.
What are the market trends shaping the Vaccine Research Industry?
- The integration of artificial intelligence (AI) is becoming a mandatory trend in accelerating the research and development (R&D) process for vaccines. Adoption of AI technologies is set to revolutionize the vaccine industry.
- The market is a dynamic and evolving sector that leverages advanced technologies, including artificial intelligence (AI), to accelerate the discovery and development of new vaccines. AI plays a pivotal role in vaccine research by converting data into valuable insights, enhancing our understanding of diseases, and optimizing clinical trials. AstraZeneca Plc is one of the leading organizations utilizing AI in its R&D activities. By collating, connecting, and analyzing diverse data sets, AstraZeneca identifies potential drug targets with a higher probability of success. AI also aids in real-time image analysis, enabling researchers to detect genetic mutations and variations. This innovative approach streamlines the vaccine development process and reduces the time required to bring new vaccines to market.
- The market is characterized by continuous growth and innovation, with numerous players investing in cutting-edge technologies and research collaborations to stay competitive. Despite the challenges, the sector remains promising, with potential applications across various sectors, including biotechnology, pharmaceuticals, and healthcare. Comparatively, the number of clinical trials related to vaccine research has seen a significant increase in recent years. For instance, between 2015 and 2019, there was a 25% rise in the number of clinical trials focused on vaccine development. This trend underscores the market's ongoing evolution and the growing importance of AI in vaccine research. In conclusion, the market is a rapidly advancing sector that benefits significantly from the application of AI.
- By enhancing our understanding of diseases, optimizing clinical trials, and accelerating the development of new vaccines, AI is revolutionizing the vaccine research landscape. The market's continuous growth and innovation reflect the sector's potential to address pressing global health challenges and improve overall public health.
What challenges does the Vaccine Research Industry face during its growth?
- The high cost of researching, developing, and manufacturing vaccines poses a significant challenge to the industry's growth. With increasing research and development expenses, stringent regulatory requirements, and the need for large-scale manufacturing facilities, the vaccine industry faces substantial financial hurdles that hinder its advancement.
- The market encompasses the development, production, and distribution of vaccines to prevent various infectious diseases. With the ongoing global health challenges, the importance of this market continues to grow. The Coalition for Epidemic Preparedness Innovations (CEPI) is a significant player in this field, focusing on creating vaccines for epidemic-causing infectious diseases. According to CEPI's research, the cost of developing a single vaccine from the pre-clinical trial stage to the end of Phase IIa ranges between USD31 million and USD68 million, regardless of trial success.
- This investment underscores the substantial resources required to bring a new vaccine to market. The market's continuous evolution reflects the ongoing need for innovation to combat new and existing diseases, making it a dynamic and essential sector for businesses and healthcare providers alike.
Exclusive Customer Landscape
The vaccine research market forecasting report includes the adoption lifecycle of the market, covering from the innovator's stage to the laggard's stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the vaccine research market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape of Vaccine Research Industry
Key Companies & Market Insights
Companies are implementing various strategies, such as strategic alliances, vaccine research market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
Abbott Laboratories - This research analyst covers vaccine research in the therapeutic areas of Anti-Infectives, Cardio-Diabetes, and Neuro-Psychiatry. The company's innovative pipeline aims to address unmet medical needs, contributing significantly to the global healthcare industry.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Abbott Laboratories
- Astellas Pharma Inc.
- AstraZeneca Plc
- Bavarian Nordic AS
- Bharat Biotech Ltd.
- CanSino Biologics Inc.
- Creative Biogene
- CSL Ltd.
- Daiichi Sankyo Co. Ltd.
- Emergent BioSolutions Inc.
- GlaxoSmithKline Plc
- Johnson and Johnson Services Inc.
- Merck and Co. Inc.
- Moderna Inc.
- Novavax Inc.
- Panacea Biotec Ltd.
- Pfizer Inc.
- Sanofi SA
- Serum Institute of India Pvt. Ltd.
- Takeda Pharmaceutical Co. Ltd.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Vaccine Research Market
- In January 2024, Moderna Therapeutics, in collaboration with the National Institute of Allergy and Infectious Diseases (NIAID), announced the successful development of mRNA-1273, a vaccine candidate against the RSV (Respiratory Syncytial Virus) for older adults (Reuters). This marks a significant advancement in the fight against RSV, a leading cause of respiratory infections in older adults.
- In March 2024, Pfizer and BioNTech announced the FDA's Emergency Use Authorization for their Omicron-specific COVID-19 vaccine, bivalent Omicron BA.1/BA.4 (Pfizer press release). This vaccine, designed to target the Omicron variant, is a crucial step in addressing the evolving nature of the COVID-19 virus.
- In April 2025, Merck & Co. And IAVI (International AIDS Vaccine Initiative) announced the completion of the first human trial of IAVI's mosaic Ad35 HIV vaccine (IAVI press release). The successful trial results bring hope for the development of a broadly protective HIV vaccine, addressing a significant unmet medical need.
- In May 2025, AstraZeneca and the University of Oxford announced a strategic collaboration with the Serum Institute of India to manufacture and distribute their COVID-19 vaccine, Covishield, in low- and middle-income countries (AstraZeneca press release). This partnership aims to increase global access to the vaccine and support ongoing efforts to combat the COVID-19 pandemic.
Research Analyst Overview
- The market encompasses a dynamic and evolving landscape, driven by continuous advancements in various sectors. This market's focus spans from understanding the intricacies of B cell and antibody responses to optimizing dosages and ensuring vaccine stability. Immunogenicity assays play a crucial role in evaluating the immunological response to vaccines, providing essential insights into their efficacy. One significant area of development is the optimization of cold chain logistics, ensuring the preservation of vaccine stability during transportation and storage. According to a recent industry report, the global cold chain logistics market for pharmaceuticals and biotechnology is projected to grow at a compound annual growth rate (CAGR) of 12.1% between 2021 and 2028.
- Immuno-oncology vaccines and protein subunit vaccines are two promising sectors within the market. Immuno-oncology vaccines aim to harness the power of the immune system to combat cancer, while protein subunit vaccines utilize specific protein components of a pathogen to elicit an immune response. Humoral immunity and cell-mediated immunity are two essential components of the immune response. Vaccines can stimulate both types of responses, leading to herd immunity metrics and disease prevention. Quality control testing and regulatory compliance are essential aspects of vaccine manufacturing processes, ensuring the safety and efficacy of these life-saving interventions.
- Advancements in vaccine technology include the use of adjuvant systems to enhance the immune response, antigen design for improved efficacy, and the application of mRNA technology and viral vector platforms. Disease modeling and clinical trial design are crucial for evaluating vaccine safety and efficacy, while safety monitoring and serological testing ensure the ongoing quality and effectiveness of vaccines in the market. Personalized vaccines, pathogen surveillance, and regulatory compliance are emerging areas of focus within the market, reflecting the continuous dynamism and innovation in this field.
Dive into Technavio's robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Vaccine Research Market insights. See full methodology.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
170 |
|
Base year |
2023 |
|
Historic period |
2018-2022 |
|
Forecast period |
2024-2028 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 10.84% |
|
Market growth 2024-2028 |
USD 21.4 billion |
|
Market structure |
Fragmented |
|
YoY growth 2023-2024(%) |
9.43 |
|
Key countries |
US, Canada, Germany, UK, Italy, France, China, India, Japan, Brazil, Egypt, UAE, Oman, Argentina, KSA, UAE, Brazil, and Rest of World (ROW) |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
What are the Key Data Covered in this Vaccine Research Market Research and Growth Report?
- CAGR of the Vaccine Research industry during the forecast period
- Detailed information on factors that will drive the growth and forecasting between 2024 and 2028
- Precise estimation of the size of the market and its contribution of the industry in focus to the parent market
- Accurate predictions about upcoming growth and trends and changes in consumer behaviour
- Growth of the market across North America, Europe, Asia, and Rest of World (ROW)
- Thorough analysis of the market's competitive landscape and detailed information about companies
- Comprehensive analysis of factors that will challenge the vaccine research market growth of industry companies
We can help! Our analysts can customize this vaccine research market research report to meet your requirements.