Varicose Vein Treatment Devices Market Size 2026-2030
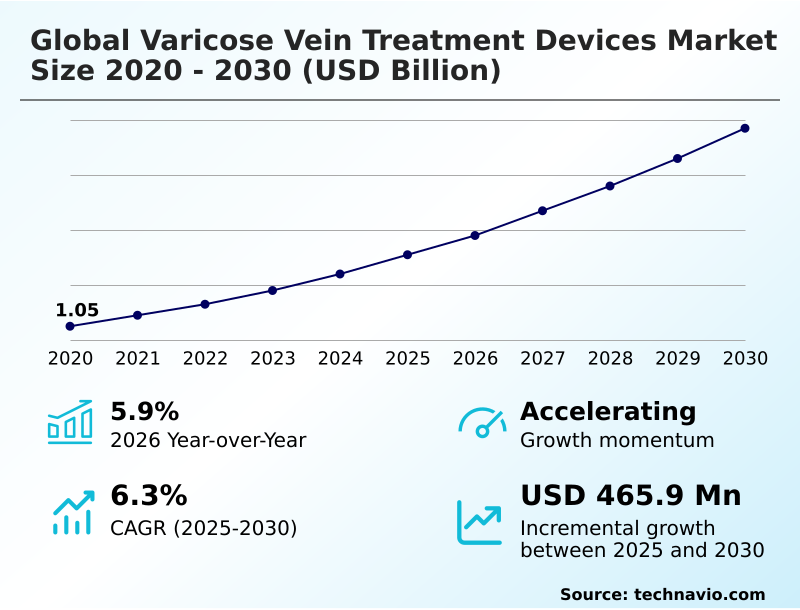
The varicose vein treatment devices market size is valued to increase by USD 465.9 million, at a CAGR of 6.3% from 2025 to 2030. Increasing geriatric population and prevalence of venous disorders will drive the varicose vein treatment devices market.
Major Market Trends & Insights
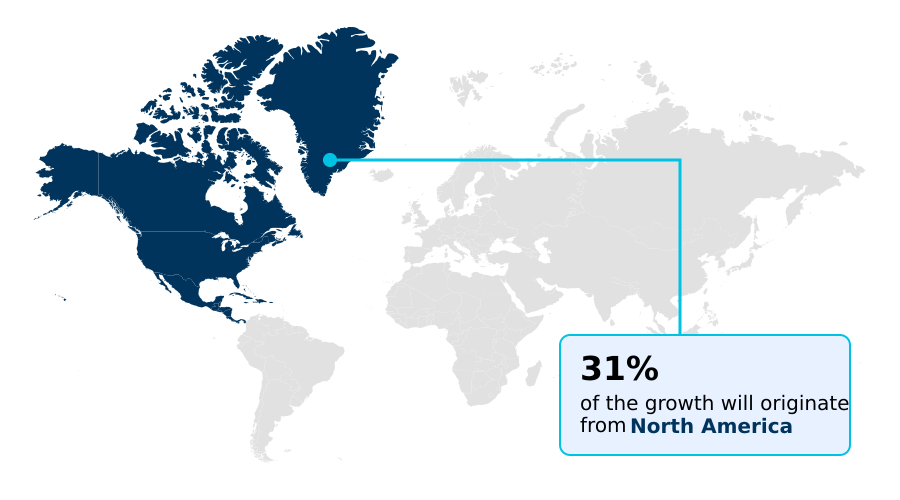
- North America dominated the market and accounted for a 31.2% growth during the forecast period.
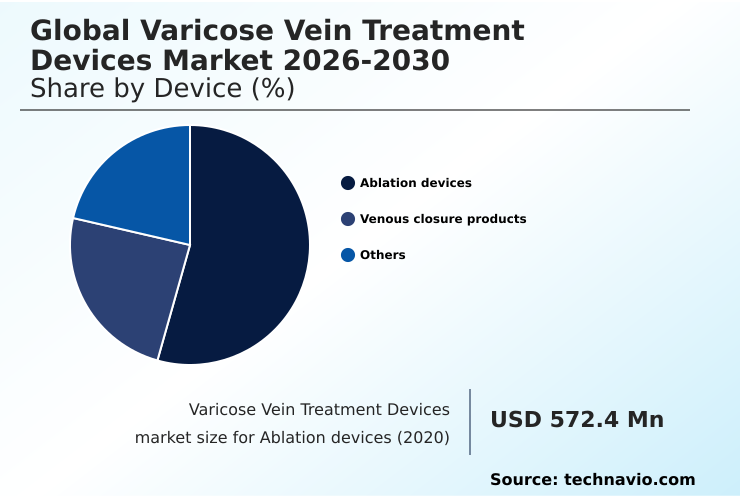
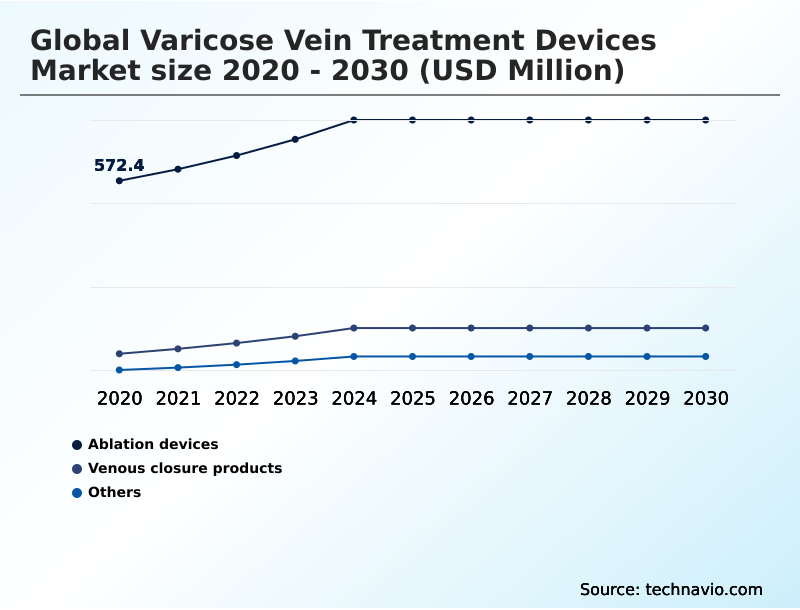
- By Device - Ablation devices segment was valued at USD 683.9 million in 2024
- By End-user - Hospitals and clinics segment accounted for the largest market revenue share in 2024
Market Size & Forecast
- Market Opportunities: USD 720.4 million
- Market Future Opportunities: USD 465.9 million
- CAGR from 2025 to 2030 : 6.3%
Market Summary
- The varicose vein treatment devices market is undergoing a significant transformation, propelled by the shift from invasive surgeries to minimally invasive techniques. Rising prevalence of venous diseases, driven by an aging population and lifestyle factors, sustains demand for effective solutions like endovenous laser ablation and radiofrequency ablation.
- A key trend is the move toward non-thermal, non-tumescent (NTNT) technologies, which reduce patient discomfort and procedural time, facilitating a migration of services to lower-cost settings like office-based laboratories and ambulatory surgical centers. However, market expansion faces headwinds from inconsistent reimbursement policies and the high upfront cost of advanced systems.
- For healthcare providers, balancing capital investment against clinical efficacy is a critical business decision.
- For example, a network of ambulatory surgical centers must conduct a thorough cost-effectiveness analysis before standardizing on a new NTNT platform, evaluating not just the device cost but also its impact on patient throughput, procedural efficiency, and the total cost of care to ensure a positive return on investment and alignment with value-based care models.
- This strategic procurement is essential for navigating the complex financial landscape while delivering superior treatment for chronic venous insufficiency.
What will be the Size of the Varicose Vein Treatment Devices Market during the forecast period?
Get Key Insights on Market Forecast (PDF) Request Free Sample
How is the Varicose Vein Treatment Devices Market Segmented?
The varicose vein treatment devices industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2026-2030, as well as historical data from 2020-2024 for the following segments.
- Device
- Ablation devices
- Venous closure products
- Others
- End-user
- Hospitals and clinics
- Ambulatory surgical centers
- Application
- Endovenous ablation
- Sclerotherapy
- Surgical ligation and stripping
- Geography
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Asia
- Rest of World (ROW)
- North America
By Device Insights
The ablation devices segment is estimated to witness significant growth during the forecast period.
Ablation devices represent a commanding segment, driven by the industry's shift to minimally invasive thermal techniques. This category, including both radiofrequency ablation and endovenous laser ablation systems, uses thermal energy for fibrosis and vessel occlusion.
Radiofrequency ablation is favored for consistent heat delivery, reducing post-procedural discomfort. Concurrently, advancements in laser diodes have improved the safety profile of endovenous laser ablation, making it highly competitive for thermal tissue ablation.
Demand is bolstered by the rising incidence of chronic venous insufficiency and a preference for office-based procedures that facilitate rapid recovery.
These methods demonstrate high efficacy, with success rates often exceeding 90%, reinforcing their status as the standard of care for truncal vein incompetence.
The Ablation devices segment was valued at USD 683.9 million in 2024 and showed a gradual increase during the forecast period.
Regional Analysis
North America is estimated to contribute 31.2% to the growth of the global market during the forecast period.Technavio’s analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
See How Varicose Vein Treatment Devices Market Demand is Rising in North America Request Free Sample
North America continues to lead the market, accounting for 31.19% of the growth, supported by its advanced healthcare infrastructure and favorable reimbursement policies for endovenous ablation.
Europe follows, with mature markets like Germany and the UK driving adoption of premium devices.
However, the most rapid expansion is occurring in Asia, which is projected to grow at a rate of 7.6%, fueled by rising disposable incomes, modernizing healthcare systems in China and India, and a burgeoning medical tourism sector in countries like Thailand.
This region is seeing a significant shift from traditional methods to minimally invasive options like sclerotherapy and laser treatments.
In contrast, the Rest of World, including South America and the Middle East, presents a mixed landscape where adoption is driven by both aesthetic demand and improving healthcare access.
Market Dynamics
Our researchers analyzed the data with 2025 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
- Navigating the varicose vein treatment landscape requires careful consideration of multiple factors, from procedural efficacy to patient recovery. A key debate involves endovenous ablation versus surgical stripping, with minimally invasive options showing a clear advantage in reducing recovery time. For instance, the typical radiofrequency ablation recovery time is days, not weeks.
- While new technologies in vein treatment are promising, they introduce new variables, such as managing the risks of cyanoacrylate glue adhesion and ensuring proper mechanochemical ablation patient selection. The role of duplex ultrasound before surgery is critical for mapping, and its use extends to improving precision with ultrasound guidance in sclerotherapy.
- Discussions around the cost of non-thermal ablation are frequent, but so is analysis of the long term success of cyanoacrylate closure. For providers, managing patient expectations involves explaining insurance coverage for venous ablation and the importance of preventing deep vein thrombosis post-ablation.
- Choosing a varicose vein specialist is crucial, as they determine the best treatment for spider veins or the suitability of laser treatment for reticular veins. They also manage sclerotherapy side effects and risks and guide patients on managing pain after endovenous laser. Procedural choices can be highly specific, such as using ambulatory phlebectomy for tortuous veins.
- Post-procedural care, like the consistent use of compression stockings after vein treatment, is vital. The effectiveness of polidocanol foam sclerotherapy for certain cases and the availability of minimally invasive options for venous ulcers further highlight the complexity and specialization within this field.
What are the key market drivers leading to the rise in the adoption of Varicose Vein Treatment Devices Industry?
- The market is primarily driven by the increasing global geriatric population and a corresponding rise in the prevalence of venous disorders.
- Market growth is primarily driven by a rising geriatric population and the increasing prevalence of venous diseases, with a substantial portion of individuals over 50 requiring intervention. This demographic trend expands the patient pool significantly.
- A second major driver is the paradigm shift toward minimally invasive techniques like endovenous laser ablation and radiofrequency ablation, which have demonstrated a capacity to increase patient throughput in outpatient clinics by over 20%.
- These advanced procedures offer reduced pain and faster recovery compared to traditional surgery. Finally, growing healthcare expenditure, favorable reimbursement policies in developed regions, and a heightened aesthetic consciousness fuel demand.
- The willingness of consumers to pay out-of-pocket for cosmetic procedures creates a resilient, dual-demand economic foundation for the market.
What are the market trends shaping the Varicose Vein Treatment Devices Industry?
- An upcoming market trend is the accelerated adoption of non-thermal and non-tumescent treatment modalities. This shift is reshaping procedural standards and patient care.
- A defining market trend is the rapid shift toward non-thermal, non-tumescent technologies, which eliminate the need for tumescent anesthesia. Modalities like cyanoacrylate adhesive closure and mechanochemical ablation are improving patient comfort, with some studies showing a reduction in post-operative pain scores by up to 40% compared to thermal methods.
- This innovation shortens total procedure time, making these treatments ideal for high-volume office-based laboratories and ambulatory surgical centers. Another key trend is the integration of advanced imaging, where augmented reality and AI-powered diagnostics enhance vein mapping precision.
- The adoption of these efficient, less invasive techniques is fundamentally altering procedural workflows, prioritizing both clinical outcomes and the patient experience, leading to higher adoption rates in developed markets.
What challenges does the Varicose Vein Treatment Devices Industry face during its growth?
- A key challenge affecting industry growth is the high cost of advanced procedures, compounded by significant variations in reimbursement policies across regions.
- Significant challenges restrain market growth, led by the high cost of advanced capital equipment and disposable devices. Initial investments in laser or radiofrequency systems can be substantial, deterring smaller clinics. This is compounded by inconsistent reimbursement policies that can limit patient access.
- Another critical challenge is the shortage of skilled practitioners, as advanced techniques require specialized training, and a lack of standardization can lead to variable patient outcomes. Furthermore, stringent regulatory frameworks imposed by bodies like the FDA and EMA require extensive clinical trials, a process that can add years to product development timelines and increase costs for manufacturers by over 30%.
- These hurdles collectively slow the adoption of new technologies, particularly in cost-sensitive and emerging markets.
Exclusive Technavio Analysis on Customer Landscape
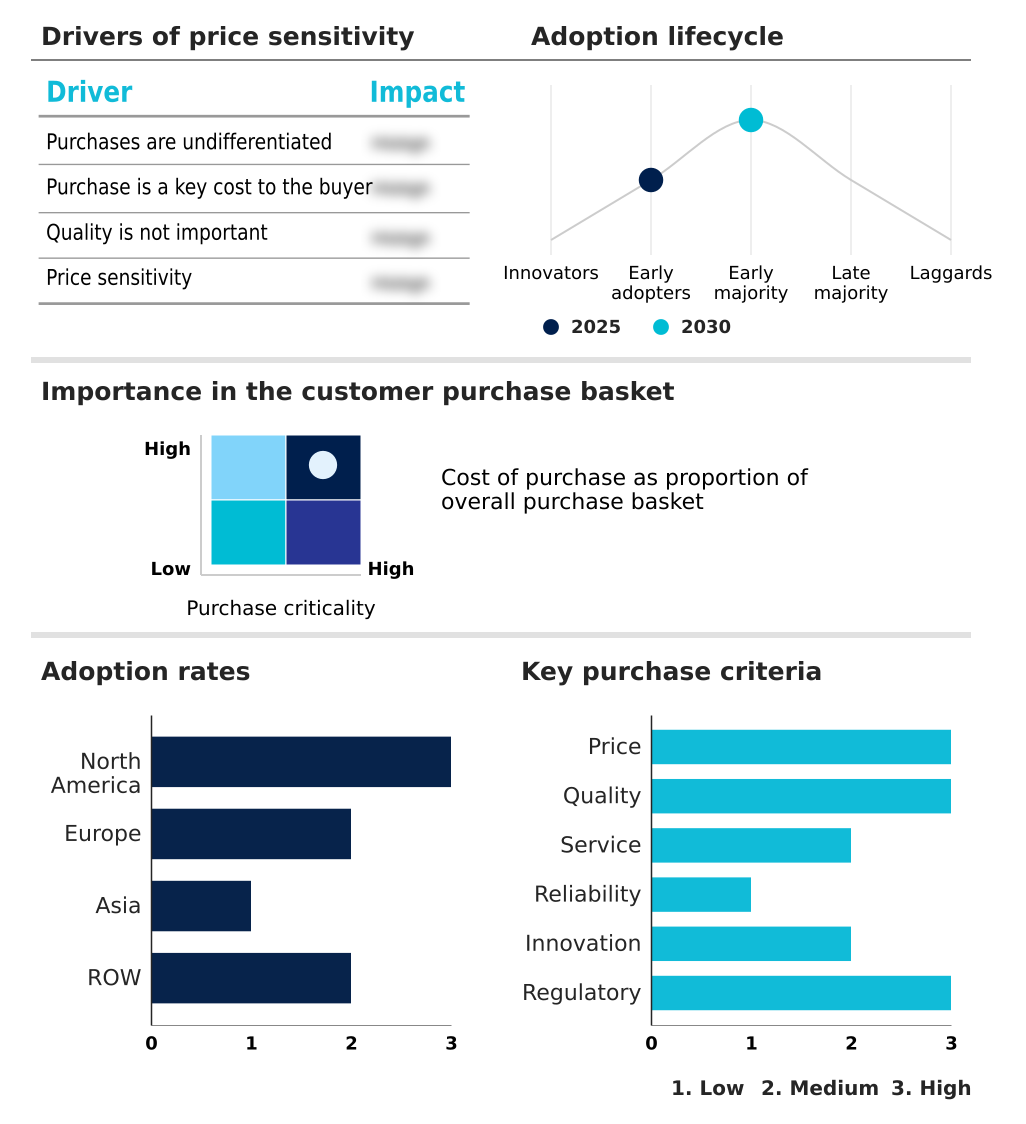
The varicose vein treatment devices market forecasting report includes the adoption lifecycle of the market, covering from the innovator’s stage to the laggard’s stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the varicose vein treatment devices market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape of Varicose Vein Treatment Devices Industry
Competitive Landscape
Companies are implementing various strategies, such as strategic alliances, varicose vein treatment devices market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
AngioDynamics Inc. - Key offerings center on minimally invasive endovenous devices, including advanced ablation and venous closure products, designed to address both therapeutic and aesthetic venous insufficiencies with improved patient outcomes.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- AngioDynamics Inc.
- Becton Dickinson and Co.
- biolitec AG
- Boston Scientific Corp.
- Candela Corp.
- Cook Group Inc.
- Cutera Inc.
- Cynosure LLC
- Dornier MedTech GmbH
- Eufoton srl
- Fotona d.o.o
- Lasotronix
- LeMaitre Vascular Inc.
- Lumenis Be Ltd.
- Medtronic Plc
- Merit Medical Systems Inc.
- Quanta System S.p.A.
- Teleflex Inc.
- Terumo Corp.
- VVT Medical
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Varicose vein treatment devices market
- In January, 2025, Stryker announced a definitive agreement to acquire Inari Medical Inc., a move intended to strengthen its footprint in the venous thromboembolism (VTE) market through Inari's leadership in peripheral vascular interventions.
- In September, 2024, THERACLION provided a strategic update on its Sonovein platform after completing its VEINRESET pivotal U.S. clinical trial, which reported a 96.8% occlusion rate, confirming the efficacy and safety of its non-invasive HIFU technology for varicose veins.
- In May, 2025, MedVasc AB, a Swedish MedTech startup, announced it had secured approximately $1 million in a funding round. The capital is allocated for the final development phase of its catheter-based device and to support preparatory activities for U.S. FDA submission.
- In February, 2025, Singapore's Health Sciences Authority granted regulatory approval for a next-generation mechanochemical ablation catheter from a leading European manufacturer, expanding access to minimally invasive options for patients with complex venous anatomies.
Dive into Technavio’s robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Varicose Vein Treatment Devices Market insights. See full methodology.
| Market Scope | |
|---|---|
| Page number | 292 |
| Base year | 2025 |
| Historic period | 2020-2024 |
| Forecast period | 2026-2030 |
| Growth momentum & CAGR | Accelerate at a CAGR of 6.3% |
| Market growth 2026-2030 | USD 465.9 million |
| Market structure | Fragmented |
| YoY growth 2025-2026(%) | 5.9% |
| Key countries | US, Canada, Mexico, Germany, UK, France, Italy, The Netherlands, Spain, Russia, China, India, Japan, South Korea, Indonesia, Thailand, Singapore, Australia, Brazil, UAE, South Africa, Saudi Arabia and Turkey |
| Competitive landscape | Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
Research Analyst Overview
- The market is defined by a rapid evolution in treating chronic venous insufficiency and vein valve incompetence, moving beyond managing symptoms toward curative peripheral vascular interventions. Boardroom decisions now center on the strategic adoption of advanced technologies versus their cost.
- While endothermal ablation, including both endovenous laser ablation and radiofrequency ablation, remains a standard for addressing saphenous vein reflux, the focus is shifting. Non-thermal non-tumescent options like cyanoacrylate adhesive closure and mechanochemical ablation are gaining traction, as they eliminate the need for tumescent anesthesia and reduce procedure times by up to 30%.
- Accurate diagnosis via duplex ultrasound guidance and advanced vein mapping technology is critical to prevent complications such as deep vein thrombosis or venous leg ulcers. For less severe cases, transdermal laser treatment, photocoagulation, and sclerotherapy using sclerosing agents like polidocanol or sodium tetradecyl sulfate are effective.
- The choice of a catheter-based device for thermal tissue ablation or a tool for ambulatory phlebectomy depends on the extent of endothelium damage and the risk of venous thromboembolism, making platform versatility a key investment criterion.
What are the Key Data Covered in this Varicose Vein Treatment Devices Market Research and Growth Report?
-
What is the expected growth of the Varicose Vein Treatment Devices Market between 2026 and 2030?
-
USD 465.9 million, at a CAGR of 6.3%
-
-
What segmentation does the market report cover?
-
The report is segmented by Device (Ablation devices, Venous closure products, and Others), End-user (Hospitals and clinics, and Ambulatory surgical centers), Application (Endovenous ablation, Sclerotherapy, and Surgical ligation and stripping) and Geography (North America, Europe, Asia, Rest of World (ROW))
-
-
Which regions are analyzed in the report?
-
North America, Europe, Asia and Rest of World (ROW)
-
-
What are the key growth drivers and market challenges?
-
Increasing geriatric population and prevalence of venous disorders, High cost of advanced procedures and reimbursement variations
-
-
Who are the major players in the Varicose Vein Treatment Devices Market?
-
AngioDynamics Inc., Becton Dickinson and Co., biolitec AG, Boston Scientific Corp., Candela Corp., Cook Group Inc., Cutera Inc., Cynosure LLC, Dornier MedTech GmbH, Eufoton srl, Fotona d.o.o, Lasotronix, LeMaitre Vascular Inc., Lumenis Be Ltd., Medtronic Plc, Merit Medical Systems Inc., Quanta System S.p.A., Teleflex Inc., Terumo Corp. and VVT Medical
-
Market Research Insights
- The market is rapidly advancing, with a focus on improving patient quality of life through minimally invasive vein treatment. The migration to outpatient venous procedures in office-based laboratories and ambulatory surgical centers has improved operational efficiency by over 25%. New technologies emphasize post-procedural pain reduction and minimize saphenous nerve injury risk.
- Innovations in aesthetic phlebology drive demand for cosmetic vein reduction, while advanced vascular access devices are crucial for treating recurrent varicose veins. The landscape includes everything from venous stent placement and vein closure systems to emerging platforms for robotic-assisted navigation and augmented reality vein visualization. The efficacy of ultrasound-guided sclerotherapy is well-documented, showing success rates above 90% in specific applications.
- However, inconsistent reimbursement for vein treatment and the need for extensive physician training for ablation remain barriers, impacting adoption despite proven benefits.
We can help! Our analysts can customize this varicose vein treatment devices market research report to meet your requirements.