Acute Ischemic Stroke Therapeutics Market Size 2026-2030
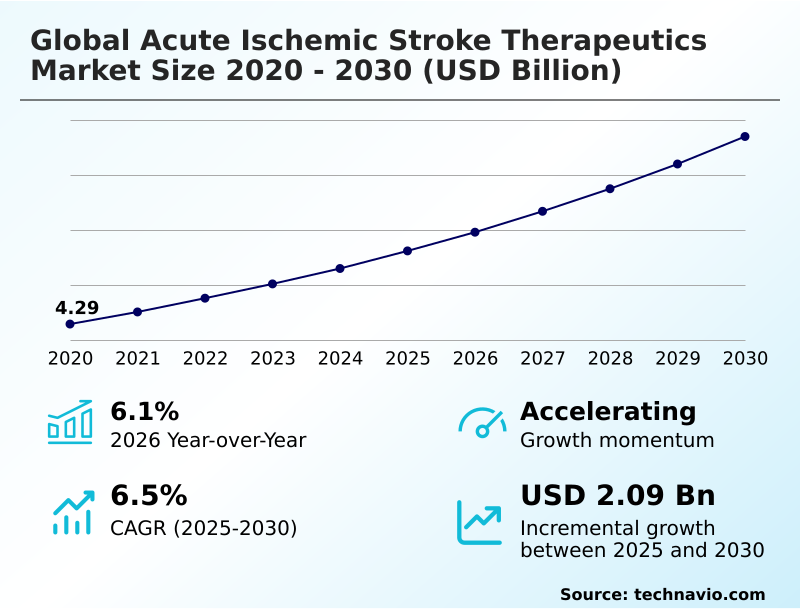
The acute ischemic stroke therapeutics market size is valued to increase by USD 2.09 billion, at a CAGR of 6.5% from 2025 to 2030. Rising prevalence of cardiovascular diseases will drive the acute ischemic stroke therapeutics market.
Major Market Trends & Insights
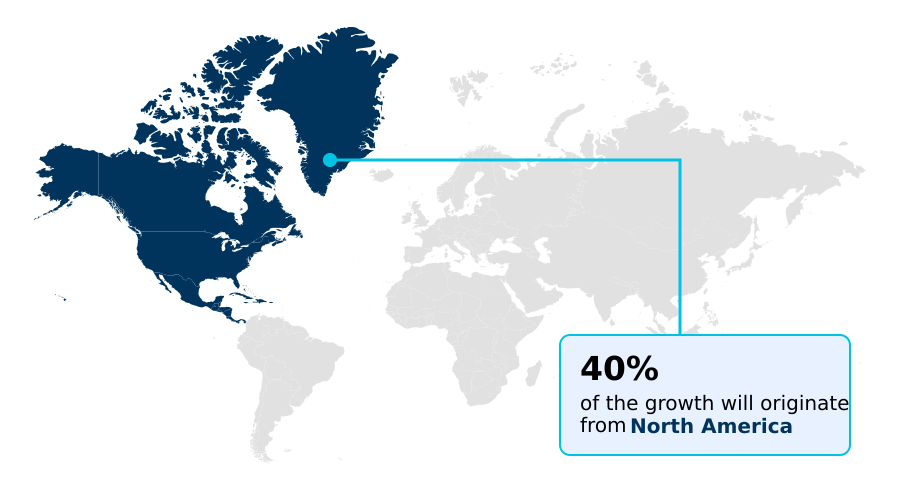
- North America dominated the market and accounted for a 40.3% growth during the forecast period.
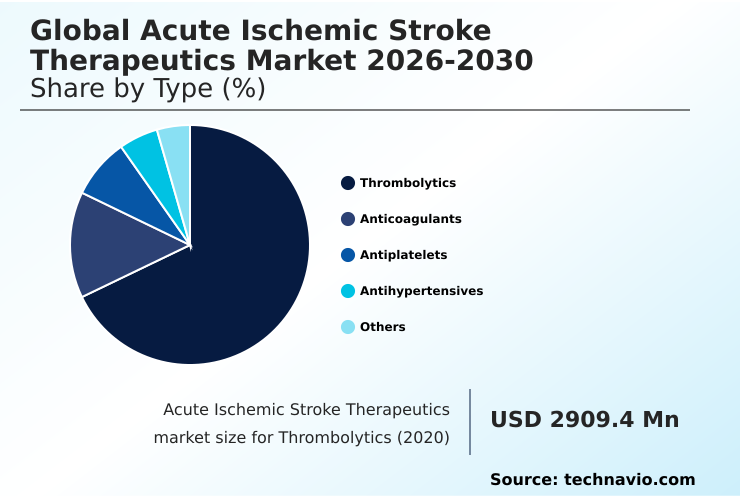
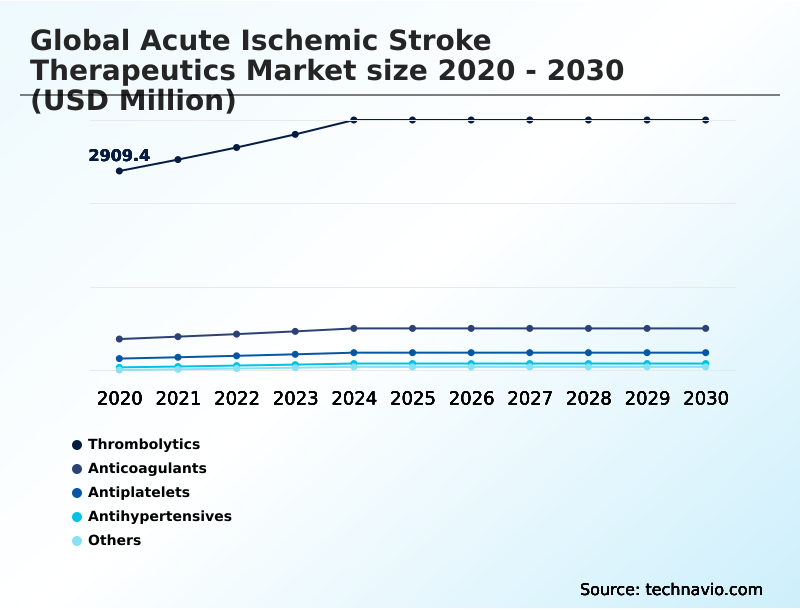
- By Type - Thrombolytics segment was valued at USD 3.61 billion in 2024
- By Distribution Channel - Hospital pharmacies segment accounted for the largest market revenue share in 2024
Market Size & Forecast
- Market Opportunities: USD 3.42 billion
- Market Future Opportunities: USD 2.09 billion
- CAGR from 2025 to 2030 : 6.5%
Market Summary
- The acute ischemic stroke therapeutics market is characterized by a dynamic shift from standardized protocols to highly personalized interventions aimed at improving patient outcomes. Core strategies focus on rapid reperfusion therapy, either through pharmacological thrombolysis or mechanical thrombectomy, to restore cerebral blood flow and salvage the ischemic penumbra.
- A key driver is the rising incidence of cardiovascular risk factors globally, which expands the at-risk population. Concurrently, the market is shaped by a strong trend toward precision medicine, where biomarker-based diagnostics and genomic risk profiling are used to tailor treatments.
- For instance, a hospital network could leverage real-world evidence analytics to evaluate the adoption of a single-bolus thrombolytic agent, projecting a 15% reduction in door-to-needle times and optimizing emergency medicine workflows. However, the market faces challenges, including the development of effective anticoagulant reversal agents and competition from advancing neurointerventional procedures.
- The robust pipeline, featuring novel factor xia inhibitors and neuroprotective peptides, signals a future of combination therapies and extended treatment windows, promising more effective and safer acute stroke management protocols.
What will be the Size of the Acute Ischemic Stroke Therapeutics Market during the forecast period?
Get Key Insights on Market Forecast (PDF) Request Free Sample
How is the Acute Ischemic Stroke Therapeutics Market Segmented?
The acute ischemic stroke therapeutics industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2026-2030, as well as historical data from 2020-2024 for the following segments.
- Type
- Thrombolytics
- Anticoagulants
- Antiplatelets
- Antihypertensives
- Others
- Distribution channel
- Hospital pharmacies
- Retail pharmacies
- Online pharmacies
- Route of administration
- Parenteral
- Oral
- Geography
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Asia
- Rest of World (ROW)
- North America
By Type Insights
The thrombolytics segment is estimated to witness significant growth during the forecast period.
The thrombolytics segment is undergoing a significant transformation, moving beyond legacy standards like tissue plasminogen activator (tpa). Recent thrombolytic therapy advancements are reshaping acute stroke management protocols by introducing agents that offer operational advantages.
This evolution directly supports more effective integration with mechanical thrombectomy for severe cases and opens new avenues for personalized stroke treatment strategies.
While foundational treatments like anticoagulant therapy and antiplatelet agents remain crucial, the focus is on optimizing the initial thrombolysis.
The development of a robust neuroprotective drug pipeline is also critical, although the immediate impact is felt from innovations in clot dissolution.
The analysis of polygenic risk scores (prs) is expected to further refine patient selection, but effective thrombolysis remains the primary intervention in the acute setting.
The Thrombolytics segment was valued at USD 3.61 billion in 2024 and showed a gradual increase during the forecast period.
Regional Analysis
North America is estimated to contribute 40.3% to the growth of the global market during the forecast period.Technavio’s analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
See How Acute Ischemic Stroke Therapeutics Market Demand is Rising in North America Request Free Sample
The geographic landscape of the acute ischemic stroke therapeutics market is led by North America, which accounts for over 40% of the incremental growth, driven by its advanced healthcare infrastructure and rapid adoption of novel treatments.
The region's leadership is reinforced by clear regulatory approval pathways and sophisticated emergency medicine workflows that facilitate therapeutic window extension. Europe follows, with highly organized stroke care networks that excel in achieving recanalization.
Meanwhile, Asia is emerging as a critical region for innovation, with significant investment in domestic R&D and dose-finding trials. This strategic focus on precision medicine and brain cytoprotection is accelerating neurovascular recovery solutions.
The development of genotype-specific drugs and combination therapy development are central to strategies in all key regions, addressing patient heterogeneity assessment and aiming to mitigate post-stroke inflammation.
Market Dynamics
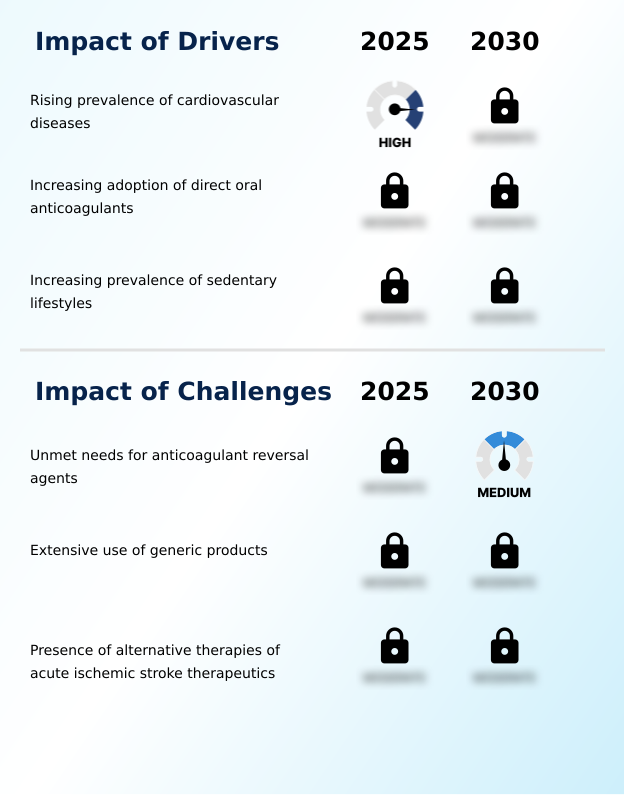
Our researchers analyzed the data with 2025 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
- The strategic landscape of the global acute ischemic stroke therapeutics market 2026-2030 is increasingly defined by the clinical and economic nuances of next-generation treatments.
- A central debate involves comparing alteplase and tenecteplase for ais, where the impact of tenecteplase on door-to-needle times is a critical factor for operational efficiency, with some comprehensive stroke centers reporting a reduction of over 15 minutes in administration protocols.
- Simultaneously, the role of factor xia inhibitors in secondary prevention is poised to revolutionize long-term care by potentially uncoupling efficacy from bleeding risk. However, this progress is moderated by persistent challenges in developing anticoagulant reversal agents.
- Advances in biomarker-driven stroke therapy selection are enabling more personalized approaches, guiding decisions on dual antiplatelet therapy duration and the use of neuroprotection strategies alongside mechanical thrombectomy. The cost-effectiveness of direct oral anticoagulants continues to influence reimbursement policies for advanced stroke therapies.
- Future growth hinges on integrating polygenic risk scores in stroke care and extending the therapeutic window for intravenous thrombolysis. The development of therapies for large vessel occlusion and strategies for enhancing collateral circulation are also key R&D priorities, with the ultimate goal of improving long-term functional outcomes after reperfusion therapy.
- The role of ai in stroke imaging and diagnosis is another critical element, helping to optimize pre-hospital stroke diagnosis and transport.
What are the key market drivers leading to the rise in the adoption of Acute Ischemic Stroke Therapeutics Industry?
- The rising global prevalence of cardiovascular diseases, which are primary risk factors for ischemic events, serves as a major driver for the acute ischemic stroke therapeutics market.
- Market growth is fundamentally driven by the urgent need for rapid cerebral blood flow restoration and the increasing prevalence of risk factors.
- Innovations in pharmacological stroke interventions are focused on improving outcomes, with dual antiplatelet therapy (dapt) protocols being refined and the efficacy of cytoprotective agents being re-evaluated as adjunctive neuroprotective therapies.
- The integration of telestroke network implementation has expanded access to care, enabling timely intravenous thrombolysis in remote regions and improving stroke rehabilitation innovations.
- This has been particularly effective in health systems where more than 40% of the population lives outside urban centers. Furthermore, research into collateral circulation enhancement and clot composition analysis is leading to better clinical trial endpoint selection.
- These efforts, combined with the seamless endovascular treatment integration, are addressing the core challenges of hemorrhagic risk mitigation and setting new standards for care.
What are the market trends shaping the Acute Ischemic Stroke Therapeutics Industry?
- The integration of genomic and clinical data via translational bioinformatics is a defining market trend. This approach leverages polygenic risk scores and multi-omics datasets to enable early risk stratification and develop personalized treatment pathways.
- Key market trends are centered on enhancing the precision and efficacy of reperfusion therapy. The development of next-generation treatments, including novel factor XIa inhibitors and neuroprotective peptides, is expanding the therapeutic arsenal beyond conventional options and creating viable intravenous alteplase alternatives.
- This innovation is supported by a deeper understanding of the ischemic penumbra, driving research into therapies that protect vulnerable brain tissue during endovascular intervention. A significant trend is the use of biomarker-based diagnostics and genomic risk profiling in stroke to tailor treatments, aligning with broader secondary stroke prevention guidelines.
- The oral anticoagulant safety profile remains a key focus, influencing the design of new agents for non-cardioembolic stroke care models. These advancements are improving pre-hospital stroke diagnosis improvements and refining multi-omics datasets for clinical use, pushing the industry toward more personalized and effective outcomes.
What challenges does the Acute Ischemic Stroke Therapeutics Industry face during its growth?
- A significant challenge impacting market growth is the persistent unmet need for rapid, effective, and widely accessible anticoagulant reversal agents.
- The market faces significant challenges in balancing innovation with the practicalities of emergency care and cost containment. While new thrombolytic agents are being developed, the extensive use of generics and the high cost of novel therapies create barriers.
- The rise of alternative treatments, such as neurointerventional procedures, has led to a 15-20% decline in intravenous thrombolytic use at some advanced centers, challenging the market position of pharmacological options. Effective secondary prevention is another hurdle, requiring optimized strategies for non-cardioembolic stroke.
- The complexity of biomarker-driven patient selection and the need for advanced ischemic core imaging to identify candidates for adjunctive therapies add operational strain. Furthermore, achieving meaningful door-to-needle time reduction remains a persistent goal. These dynamics underscore the pressure to align with value-based care models and demonstrate clear advantages in penumbral salvage techniques to secure adoption.
Exclusive Technavio Analysis on Customer Landscape
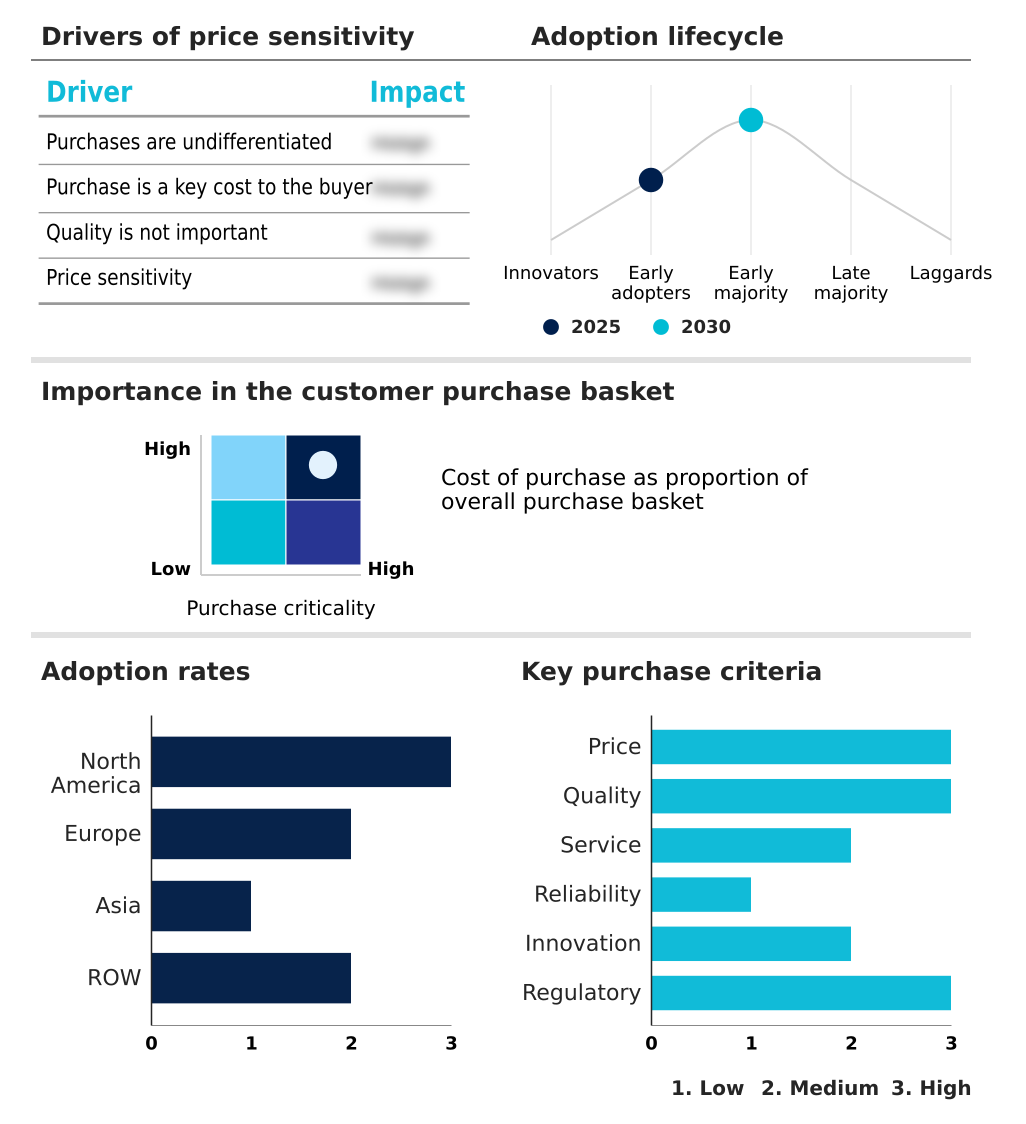
The acute ischemic stroke therapeutics market forecasting report includes the adoption lifecycle of the market, covering from the innovator’s stage to the laggard’s stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the acute ischemic stroke therapeutics market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape of Acute Ischemic Stroke Therapeutics Industry
Competitive Landscape
Companies are implementing various strategies, such as strategic alliances, acute ischemic stroke therapeutics market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
Amgen Inc. - Key offerings focus on discovering, developing, and delivering innovative human therapeutics, primarily targeting cardiovascular and metabolic diseases to advance patient care.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Amgen Inc.
- Anthos Therapeutics
- AstraZeneca Plc
- Bayer AG
- Boehringer Ingelheim GmbH
- Bristol Myers Squibb Co.
- Daiichi Sankyo Co. Ltd.
- F. Hoffmann La Roche Ltd.
- HEALIOS K.K.
- Johnson and Johnson Services
- Medtronic Plc
- Merck and Co. Inc.
- NoNO Inc.
- Pfizer Inc.
- Remedy Pharmaceuticals
- Sanofi SA
- Stryker Corp.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Acute ischemic stroke therapeutics market
- In August 2024, Simcere Pharmaceutical Group received Breakthrough Therapy Designation from the U.S. FDA for Sanbexin, a sublingual formulation of edaravone dexborneol intended to preserve brain tissue during acute ischemia.
- In March 2025, F. Hoffmann-La Roche Ltd. (Genentech) obtained U.S. FDA approval for Tenecteplase to treat acute ischemic stroke, marking the first new thrombolytic agent approved for this indication in nearly three decades.
- In May 2025, Medtronic Plc formed a strategic partnership with Brainomix, integrating advanced AI software with its neurovascular devices to enhance patient selection for mechanical thrombectomy procedures.
- In May 2025, Merck KGaA broadened its neurology portfolio by acquiring the rights to ApTOLL from aptaTargets, a strategic move aimed at accelerating the development of novel therapies to mitigate brain damage after a stroke.
Dive into Technavio’s robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Acute Ischemic Stroke Therapeutics Market insights. See full methodology.
| Market Scope | |
|---|---|
| Page number | 289 |
| Base year | 2025 |
| Historic period | 2020-2024 |
| Forecast period | 2026-2030 |
| Growth momentum & CAGR | Accelerate at a CAGR of 6.5% |
| Market growth 2026-2030 | USD 2086.7 million |
| Market structure | Fragmented |
| YoY growth 2025-2026(%) | 6.1% |
| Key countries | US, Canada, Mexico, Germany, UK, France, Italy, Spain, The Netherlands, China, Japan, India, Indonesia, South Korea, Thailand, Brazil, South Africa, UAE, Saudi Arabia, Turkey, Israel, Argentina and Colombia |
| Competitive landscape | Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
Research Analyst Overview
- The acute ischemic stroke therapeutics market is undergoing a fundamental restructuring, pivoting from broad-spectrum interventions to high-precision strategies. This evolution is driven by advances in thrombolysis and neuroprotection, where the goal is to maximize reperfusion therapy efficacy while mitigating hemorrhagic risk.
- The approval of new thrombolytic agents with superior fibrin specificity is enabling a significant reduction in door-to-needle times, with some health systems achieving a 20% improvement in administration efficiency. This operational gain allows for better integration of adjunctive therapies and mechanical thrombectomy for endovascular intervention.
- A key boardroom-level consideration is the allocation of R&D investment toward biomarker-based diagnostics and multi-omics datasets to enable true precision medicine. The pipeline is rich with factor xia inhibitors and neuroprotective peptides designed to enhance collateral circulation and protect the ischemic penumbra.
- Success requires a deep understanding of clot composition analysis, polygenic risk scores (prs), and the mechanisms of post-stroke inflammation. Future strategies will center on combination therapy development, therapeutic window extension, and brain cytoprotection to achieve meaningful neurovascular recovery and improve outcomes in non-cardioembolic stroke.
- The development of effective cytoprotective agents and advancements in dual antiplatelet therapy (dapt) for secondary prevention are also critical for market positioning.
What are the Key Data Covered in this Acute Ischemic Stroke Therapeutics Market Research and Growth Report?
-
What is the expected growth of the Acute Ischemic Stroke Therapeutics Market between 2026 and 2030?
-
USD 2.09 billion, at a CAGR of 6.5%
-
-
What segmentation does the market report cover?
-
The report is segmented by Type (Thrombolytics, Anticoagulants, Antiplatelets, Antihypertensives, and Others), Distribution Channel (Hospital pharmacies, Retail pharmacies, and Online pharmacies), Route of Administration (Parenteral, and Oral) and Geography (North America, Europe, Asia, Rest of World (ROW))
-
-
Which regions are analyzed in the report?
-
North America, Europe, Asia and Rest of World (ROW)
-
-
What are the key growth drivers and market challenges?
-
Rising prevalence of cardiovascular diseases, Unmet needs for anticoagulant reversal agents
-
-
Who are the major players in the Acute Ischemic Stroke Therapeutics Market?
-
Amgen Inc., Anthos Therapeutics, AstraZeneca Plc, Bayer AG, Boehringer Ingelheim GmbH, Bristol Myers Squibb Co., Daiichi Sankyo Co. Ltd., F. Hoffmann La Roche Ltd., HEALIOS K.K., Johnson and Johnson Services, Medtronic Plc, Merck and Co. Inc., NoNO Inc., Pfizer Inc., Remedy Pharmaceuticals, Sanofi SA and Stryker Corp.
-
Market Research Insights
- The market dynamics for acute ischemic stroke therapeutics are increasingly influenced by the integration of advanced technologies and personalized medicine. The focus on secondary stroke prevention guidelines and tailored non-cardioembolic stroke care models is driving innovation beyond initial treatment.
- A notable shift is seen in the adoption of digital health tools, with telemedicine utilization for stroke care surging by 40% and AI-powered diagnostic adoption growing by 35%. This push is complemented by advances in pharmacological stroke interventions, where research into adjunctive neuroprotective therapies and improvements in pre-hospital stroke diagnosis are creating new value.
- As genomic-based therapeutics see a 25% year-over-year expansion, the emphasis on patient heterogeneity assessment and genotype-specific drugs is reshaping R&D priorities, steering the industry toward more precise and effective acute stroke management protocols.
We can help! Our analysts can customize this acute ischemic stroke therapeutics market research report to meet your requirements.