US Durable Medical Equipment Market Size 2024-2028
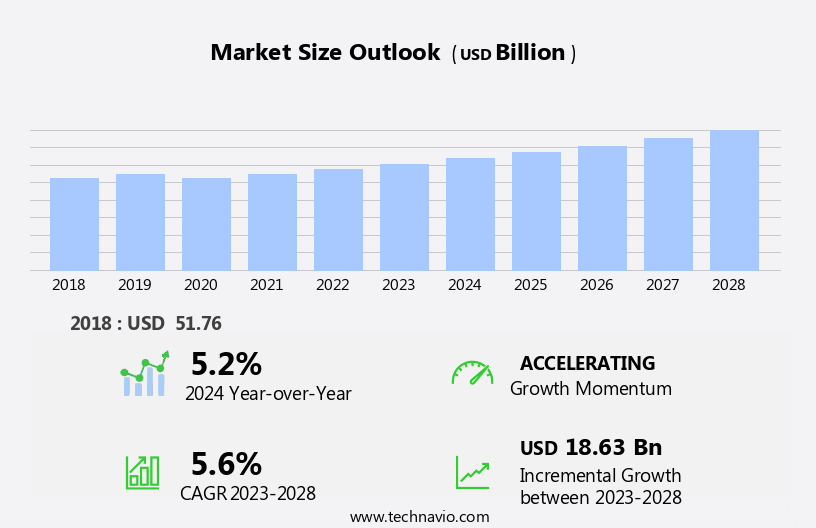
The US durable medical equipment market size is forecast to increase by USD 18.63 billion, at a CAGR of 5.6% between 2023 and 2028.
- The Durable Medical Equipment (DME) market in the US is experiencing significant growth, driven by the expanding geriatric population and increasing patient preference for at-home medical treatment. The aging demographic trend is fueling demand for DME as the elderly population often requires more medical equipment and supplies to maintain their health and independence. Furthermore, the convenience and cost savings associated with at-home care are leading more patients to opt for DME solutions instead of institutionalized care. However, the DME market faces challenges that could hinder its growth. The high cost of durable medical equipment is a significant obstacle, making it difficult for some patients to afford necessary devices. Popular categories within the DME market include power scooters, oxygen equipment, walkers, wheelchairs, hospital beds, catheters, support braces, CPAP masks, and various other medical devices used in the treatment of conditions such as cancer and cardiac disorders.
- Additionally, regulatory compliance and reimbursement policies pose challenges for market participants, requiring them to navigate complex regulations and ensure proper documentation to secure payment for their products and services. Companies seeking to capitalize on market opportunities must address these challenges by offering affordable pricing models, focusing on regulatory compliance, and providing exceptional customer service to ensure patient satisfaction and repeat business.
What will be the size of the US Durable Medical Equipment Market during the forecast period?
Explore in-depth regional segment analysis with market size data - historical 2018-2022 and forecasts 2024-2028 - in the full report.
Request Free Sample
- The Durable Medical Equipment (DME) market in the US is characterized by a focus on medical device safety, quality assurance systems, and regulatory compliance. Patient education materials and risk management strategies are essential components of ensuring safe and effective use of DME. Medical device testing and data privacy regulations play a critical role in maintaining trust with patients and healthcare providers. Wearable medical sensors and device interoperability are driving innovation in the DME sector, enabling better patient outcome measures and value-based healthcare. Medical equipment rental, including oxygen concentrators and home infusion therapy, offers cost-effective solutions for patients requiring long-term care.
- Supply chain efficiency and infection control measures are key priorities for DME providers, with performance improvement initiatives and clinical documentation essential for maintaining regulatory compliance. Assistive technology, such as insulin pumps and wheelchair technology, is transforming the way patients manage their conditions at home. Medical device repair and cost-effectiveness studies are important considerations for healthcare providers seeking to optimize their DME investments. CPAP machines, enteral feeding pumps, bariatric equipment, and hospital bed systems are all subject to ongoing research and development to improve performance and patient satisfaction. Pressure ulcer prevention and medication adherence are critical areas of focus for DME providers, with equipment tracking systems and infection control measures essential for maintaining patient safety and quality of care.
How is this market segmented?
The market research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD billion" for the period 2024-2028, as well as historical data from 2018-2022 for the following segments.
- Product Type
- Monitoring and therapeutic devices
- Personal mobility devices
- Bathroom safety devices and medical furniture
- End-user
- Hospitals and clinics
- Home healthcare
- Ambulatory surgical centers
- Geography
- North America
- US
- North America
By Product Type Insights
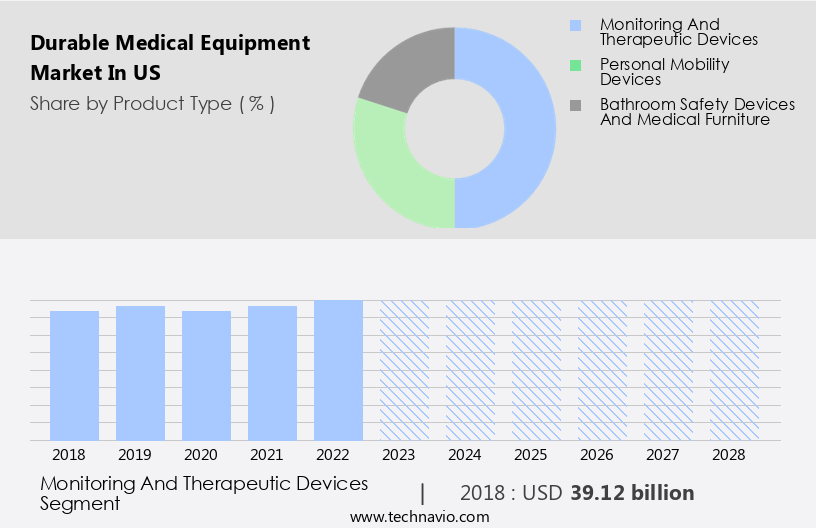
The monitoring and therapeutic devices segment is estimated to witness significant growth during the forecast period.
In the dynamic and evolving landscape of medical devices, various sectors are experiencing significant growth and innovation. Orthopedic implants continue to advance, offering improved patient outcomes through enhanced materials and design. Data analytics in healthcare is revolutionizing diagnostics and treatment plans, enabling more personalized care. Sleep apnea therapy devices are gaining traction, addressing the growing prevalence of sleep disorders. Equipment lifecycle management is increasingly important, ensuring optimal performance and reducing healthcare costs. Diagnostic imaging devices, from MRI to CT scans, are essential tools in early detection and treatment. Patient satisfaction metrics are prioritized, with remote patient monitoring and telehealth integration improving accessibility and convenience.
Surgical instruments and medical equipment sterilization are crucial for patient safety, while home healthcare technology and medical equipment financing offer greater access to care. Prosthetic limbs, clinical trial devices, and rehabilitation equipment are transforming mobility and recovery. Wound care management and incontinence care products cater to the needs of vulnerable populations. Reimbursement processes and healthcare provider networks are streamlined through clinical workflow optimization. Patient monitoring systems, including cardiac and respiratory devices, are essential for continuous care. Diabetes management devices and mobility assistance devices are improving the quality of life for millions. Supply chain logistics are optimized through technology, ensuring the timely delivery of essential medical equipment.
Device cybersecurity is a growing concern, with insurance coverage and durable medical supplies playing crucial roles in accessibility and affordability. Rehabilitation equipment, patient monitoring systems, and reimbursement processes are all interconnected, shaping the future of healthcare.
The Monitoring and therapeutic devices segment was valued at USD 39.12 billion in 2018 and showed a gradual increase during the forecast period.
Market Dynamics
Our researchers analyzed the data with 2023 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
What are the US Durable Medical Equipment Market drivers leading to the rise in adoption of the Industry?
- The geriatric population's continued growth serves as the primary market driver.
- The global aging population, particularly in countries like the US and Canada, is experiencing a significant increase, with approximately 17% aged above 65 years in 2019. This demographic trend is driving the growth of the durable medical equipment market. The leading causes of death among the elderly include heart disease, cancer, and chronic respiratory diseases. Consequently, there is a growing demand for patient monitoring systems, incontinence care products, respiratory therapy equipment, diabetes management devices, and mobility assistance devices. Healthcare provider networks are integrating telehealth services to enhance patient care and streamline reimbursement processes.
- The integration of telehealth into durable medical equipment is expected to further fuel market growth. These factors collectively contribute to the expansion of the durable medical equipment market.
What are the US Durable Medical Equipment Market trends shaping the Industry?
- The preference for at-home medical treatments is growing increasingly popular in the current market trend. This shift towards in-home healthcare solutions is a significant development in the healthcare industry.
- Home healthcare equipment plays a crucial role in enabling individuals to receive medical care in the comfort of their own homes. This trend is particularly significant for those with chronic conditions and the elderly population. The demand for home healthcare devices is driven by several factors, including the increasing prevalence of chronic diseases and the growing geriatric population. These devices include vital signs monitors, activity monitors, sleep monitors, and other equipment. Moreover, advancements in medical device regulation, data analytics in healthcare, and remote patient monitoring have further fueled the growth of this market. For instance, diagnostic imaging devices and orthopedic implants can now be monitored and analyzed remotely, ensuring timely intervention and improved patient outcomes.
- Sleep apnea therapy is another area where home healthcare equipment has gained significant traction, with the availability of non-invasive devices and remote monitoring capabilities. Equipment lifecycle management is another critical aspect of the home healthcare market. Effective management of the entire lifecycle of medical devices, from procurement to disposal, is essential to ensure patient safety and cost-effectiveness. Patient satisfaction metrics are also increasingly being used to evaluate the performance of home healthcare devices and services, further driving market growth. The healthcare industry's shift towards home healthcare has been a notable trend in recent years, with many people preferring the convenience and comfort of receiving care at home.
How does US Durable Medical Equipment Market face challenges during its growth?
- The escalating costs of durable medical equipment pose a significant challenge to the industry's growth trajectory.
- The durable medical equipment market encompasses essential devices used in healthcare facilities for diagnosing and treating various conditions. Due to their high cost, many end-users opt for leasing instead of purchasing new equipment. This financing solution allows for reduced maintenance and installation expenses, making it an attractive option for healthcare providers. For instance, the expense of acquiring a digital mammography system is substantial. However, the increasing prevalence of breast cancer necessitates access to advanced medical imaging technology. Leasing providers offer favorable terms for medical imaging equipment, enabling healthcare facilities to access cutting-edge technology while managing costs effectively. Furthermore, home healthcare technology, such as prosthetic limbs and medical equipment sterilization systems, are crucial for patient safety protocols.
- Regular equipment maintenance is essential to ensure optimal functionality and patient safety. By leasing equipment, healthcare providers can allocate resources towards maintaining these essential devices, ultimately contributing to overall cost reduction in the industry.
Exclusive US Durable Medical Equipment Market Customer Landscape
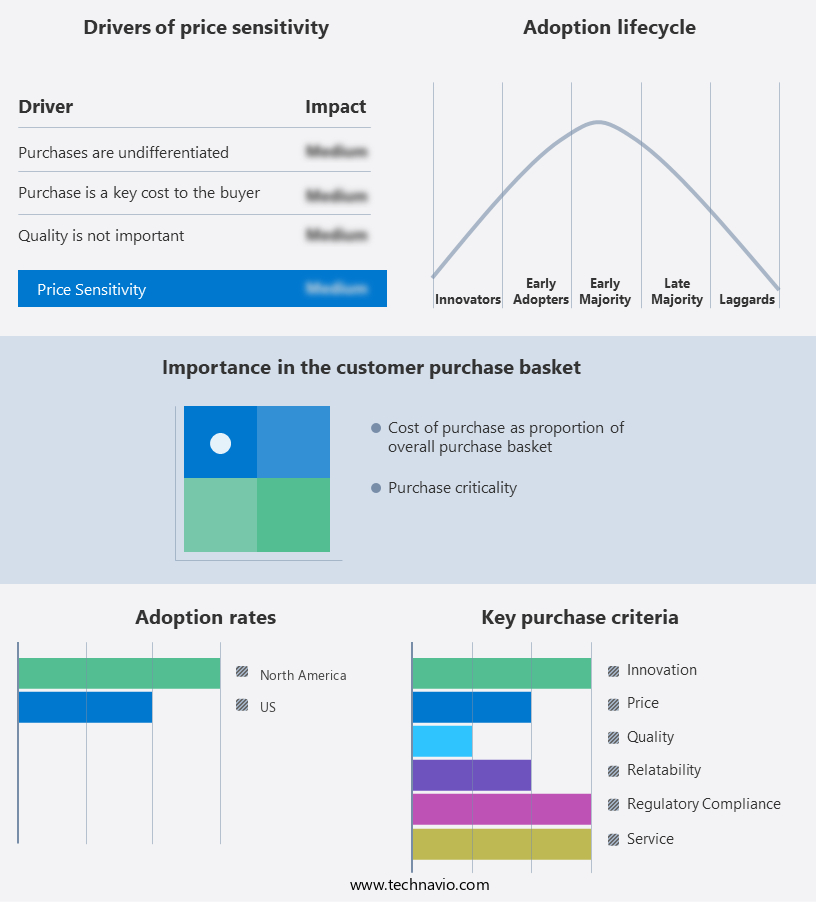
The market forecasting report includes the adoption lifecycle of the market, covering from the innovator's stage to the laggard's stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape
Key Companies & Market Insights
Companies are implementing various strategies, such as strategic alliances, market forecast partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the market.
The market research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Arjo AB
- Baxter International Inc.
- Becton Dickinson and Co.
- Cardinal Health Inc.
- Clear Image Devices LLC
- Compass Health Brands
- GE Healthcare Technologies Inc.
- GF Health Products Inc.
- Hill Rom Holdings Inc.
- Invacare Corp.
- Joerns Healthcare LLC
- Kaye Products Inc.
- Medical Device Depot Inc.
- Medline Industries LP
- NewLeaf Health LLC
- NOVA Medical Products
- SOMA TECH INTL
- Stryker Corp.
- Sunrise Medical LLC
- TZ Medical
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key market players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Durable Medical Equipment Market In US
- In January 2024, Medtronic, a leading medical technology company, announced the FDA approval of its new insulin pump system, MiniMedTM 780G, integrating advanced hybrid closed-loop technology to improve diabetes management for patients (Medtronic Press Release, 2024).
- In March 2024, ResMed, a global leader in sleep apnea devices, entered into a strategic partnership with Philips, a Dutch technology company, to expand its product offerings in the sleep apnea market through the integration of Philips' Blueventure sleep and respiratory care solutions (ResMed Press Release, 2024).
- In April 2025, Kindred Healthcare, a leading provider of healthcare services, completed the acquisition of Gentiva Health Services, a significant home health and hospice care provider, strengthening its presence in the home healthcare market and expanding its reach to over 1,600 locations (Kindred Healthcare Press Release, 2025).
- In May 2025, FDA granted clearance to Becton, Dickinson and Company for its new remote patient monitoring system, StrokeGuard RPM, designed to detect and monitor stroke risk factors in real-time, marking a significant advancement in telehealth technology for stroke care (BD Press Release, 2025).
Research Analyst Overview
The durable medical equipment (DME) market in the US is characterized by continuous evolution and dynamic market activities. Medical device regulation plays a pivotal role in shaping the industry, ensuring the safety and efficacy of various DME offerings. Orthopedic implants, a significant segment, witness ongoing advancements in materials and designs. Data analytics in healthcare is increasingly being integrated into DME, enabling more effective equipment utilization and patient care. Sleep apnea therapy, a growing application, benefits from technological innovations and an expanding patient base. Equipment lifecycle management is gaining importance, with providers focusing on optimizing asset utilization and reducing costs.
Diagnostic imaging devices continue to advance, offering enhanced accuracy and accessibility. Patient satisfaction metrics are increasingly influencing purchasing decisions, driving competition among DME providers. Remote patient monitoring is transforming healthcare delivery, enabling real-time patient data collection and analysis. Surgical instruments, medical equipment sterilization, home healthcare technology, medical equipment financing, and patient safety protocols are other key areas of focus. The integration of telehealth, rehabilitation equipment, and diabetes management devices is revolutionizing care delivery. DME providers are also addressing supply chain logistics, device cybersecurity, insurance coverage, and reimbursement processes to meet evolving market demands. In the realm of clinical trials, DME plays a crucial role in optimizing workflows and ensuring data integrity.
Wound care management, incontinence care products, and rehabilitation equipment cater to specific patient needs. Prosthetic limbs and mobility assistance devices enable improved patient mobility and independence. The DME market is a complex ecosystem, with various stakeholders influencing its dynamics. As the industry continues to evolve, providers must adapt to changing market conditions and regulatory requirements to remain competitive.
Dive into Technavio's robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Durable Medical Equipment Market in US insights. See full methodology.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
118 |
|
Base year |
2023 |
|
Historic period |
2018-2022 |
|
Forecast period |
2024-2028 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 5.6% |
|
Market growth 2024-2028 |
USD 18.63 billion |
|
Market structure |
Fragmented |
|
YoY growth 2023-2024(%) |
5.2 |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
What are the Key Data Covered in this Market Research Report?
- CAGR of the market during the forecast period
- Detailed information on factors that will drive the market growth and forecasting between 2024 and 2028
- Precise estimation of the size of the market and its contribution of the market in focus to the parent market
- Accurate predictions about upcoming market growth and trends and changes in consumer behaviour
- Growth of the market across US
- Thorough analysis of the market's competitive landscape and detailed information about companies
- Comprehensive analysis of factors that will challenge the growth of market companies
We can help! Our analysts can customize this market research report to meet your requirements Get in touch