Pharmaceutical Intermediates Market Market Size 2026-2030
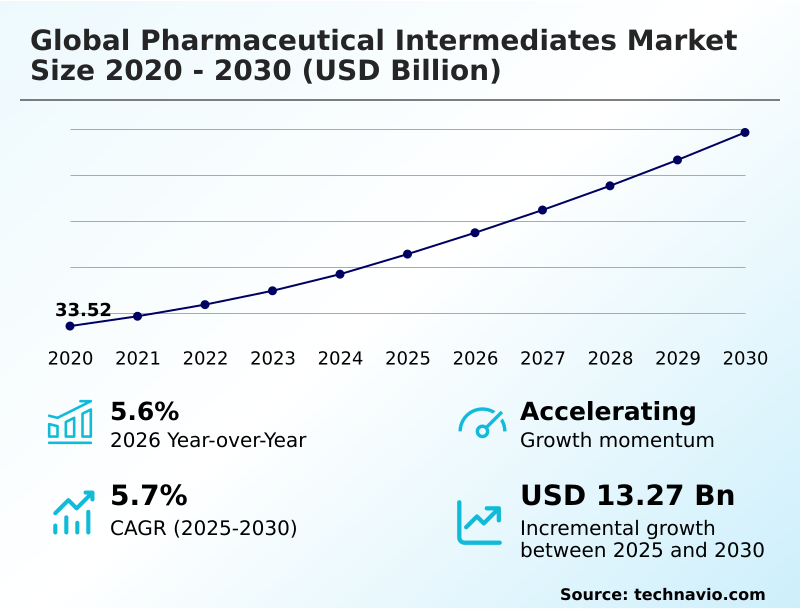
The pharmaceutical intermediates market market size is valued to increase by USD 13.27 billion, at a CAGR of 5.7% from 2025 to 2030. Rising prevalence of chronic diseases and aging global population will drive the pharmaceutical intermediates market market.
Major Market Trends & Insights
- Asia dominated the market and accounted for a 38% growth during the forecast period.
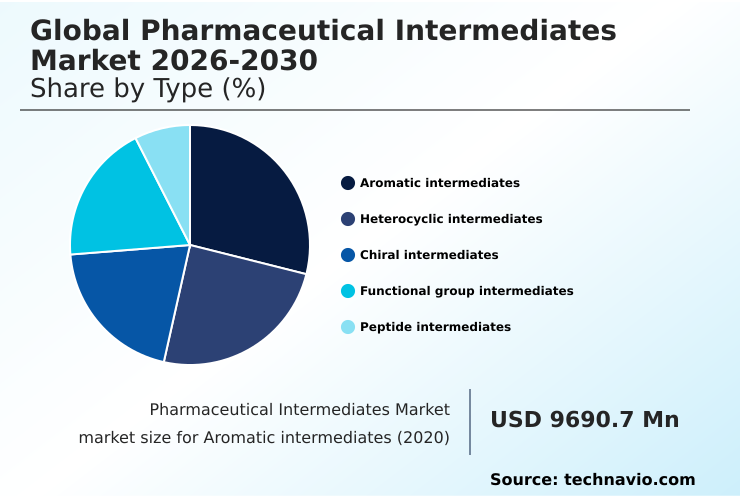
- By Type - Aromatic intermediates segment was valued at USD 11.20 billion in 2024
- By Application - Anti-inflammatory drugs segment accounted for the largest market revenue share in 2024
Market Size & Forecast
- Market Opportunities: USD 21.12 billion
- Market Future Opportunities: USD 13.27 billion
- CAGR from 2025 to 2030 : 5.7%
Market Summary
- The pharmaceutical intermediates market is foundational to the broader pharmaceutical industry, providing the essential molecular building blocks required for synthesizing active pharmaceutical ingredients (APIs). Growth is underpinned by the rising global prevalence of chronic diseases and an aging population, which sustains high-volume demand for a wide array of therapeutics.
- Concurrently, the burgeoning generic drugs industry creates immense, volume-driven demand as blockbuster drugs lose patent protection, intensifying the need for cost-effective intermediate supply chains. A key business scenario involves an innovative biotech firm deciding to forgo capital-intensive in-house manufacturing.
- Instead, it engages a specialized contract development and manufacturing organization (CDMO) to handle the complex, multi-step synthesis of its proprietary chiral intermediates. This outsourcing strategy allows the firm to accelerate its drug development timeline and focus its resources on core R&D and clinical trials.
- However, the market is not without complexities; manufacturers must navigate a labyrinth of stringent cGMP regulations and address vulnerabilities in a supply chain heavily concentrated in Asia, all while facing pressure to adopt greener, more sustainable chemical processes. The industry's trajectory is toward more complex, high-value molecules, requiring advanced synthetic expertise.
What will be the Size of the Pharmaceutical Intermediates Market Market during the forecast period?
Get Key Insights on Market Forecast (PDF) Request Free Sample
How is the Pharmaceutical IntermediatesMarket Market Segmented?
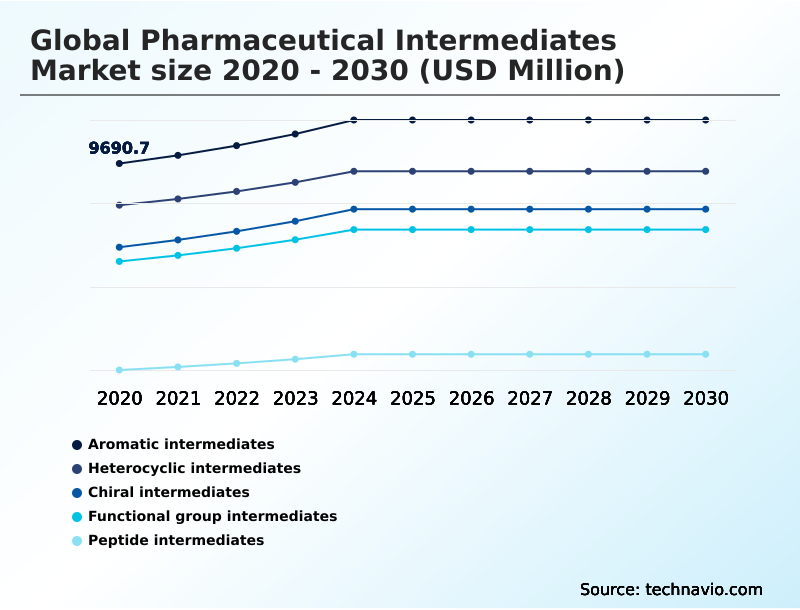
The pharmaceutical intermediatesmarket industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2026-2030, as well as historical data from 2020-2024 for the following segments.
- Type
- Aromatic intermediates
- Heterocyclic intermediates
- Chiral intermediates
- Functional group intermediates
- Peptide intermediates
- Application
- Anti-inflammatory drugs
- Analgesics
- Cardiovascular drugs
- Anti cancer drugs
- Others
- End-user
- Biotech and pharma companies
- CMOs and CROs
- Research laboratory
- Geography
- North America
- US
- Canada
- Mexico
- Asia
- Europe
- Germany
- UK
- France
- Rest of World (ROW)
- North America
By Type Insights
The aromatic intermediates segment is estimated to witness significant growth during the forecast period.
Aromatic intermediates represent a foundational class of organic compounds, serving as the core scaffold for a vast number of small-molecule active pharmaceutical ingredients (APIs).
Their demand is primarily driven by the high-volume production of established generic drugs, where cost efficiency, purity, and supply chain reliability are paramount.
This segment is characterized by intense competition, compelling manufacturers to focus on operational excellence and economies of scale. Key trends involve the development of greener synthesis routes to minimize environmental impact.
The strategic importance of these molecules as workhorse chemical building blocks is undeniable; their stable, cost-effective supply is essential for producing a significant portion of the world's medicines, with some widely used analgesics seeing a 15% improvement in yield through advanced process chemistry.
The Aromatic intermediates segment was valued at USD 11.20 billion in 2024 and showed a gradual increase during the forecast period.
Regional Analysis
Asia is estimated to contribute 38% to the growth of the global market during the forecast period.Technavio’s analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
See How Pharmaceutical IntermediatesMarket Market Demand is Rising in Asia Request Free Sample
The global geographic landscape is defined by a strategic dichotomy. Asia, particularly China and India, remains the manufacturing powerhouse, accounting for over 38% of the market's incremental growth, driven by its vast infrastructure for producing fine chemicals and starting materials.
These nations are pivotal intermediate chemical suppliers for the global drug substance manufacturing ecosystem. However, significant supply chain vulnerabilities have prompted a counter-trend in North America and Europe, where reshoring and regionalization initiatives are gaining momentum.
Supported by government policies, US and European CDMOs are expanding their capacity for advanced intermediates and API synthesis.
This strategic shift aims to enhance pharmaceutical supply chain resilience, as reliance on single-source regions previously led to production delays for up to 45% of surveyed companies, underscoring the drive for a more balanced and secure global network.
Market Dynamics
Our researchers analyzed the data with 2025 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
- Strategic planning in the global pharmaceutical intermediates market 2026-2030 requires a multi-faceted approach to navigate a complex and competitive environment. Boardroom decisions increasingly center on the total cost of chiral intermediates production, not just the unit price, factoring in the long-term value of quality and supply security.
- Adherence to evolving GMP guidelines for API intermediates is non-negotiable, demanding continuous investment in quality systems and advanced analytics for purity profiling. Companies are actively exploring the benefits of continuous manufacturing for intermediates, with flow chemistry in intermediate synthesis offering significant advantages in safety, consistency, and footprint reduction.
- This has made api synthesis route scouting services that incorporate such technologies highly valuable. The trend of outsourcing advanced intermediate manufacturing services continues, as it provides access to specialized expertise in areas like high-potency API handling. This allows firms to focus on core competencies while managing pharmaceutical supply chain vulnerabilities by diversifying their partner base.
- Securing reliable starting material suppliers is a critical risk mitigation in drug substance manufacturing. Decision-makers are also comparing CDMO capabilities for peptide synthesis to capitalize on the booming biologics sector, while technology transfer for intermediate processes must be seamless to maintain timelines.
- As companies navigate patent cliffs for intermediates, the ability to rapidly scale production of building blocks for generic drugs becomes a key competitive differentiator, with some achieving a 20% faster scale-up than competitors. This entire framework depends on a robust strategy for sustainability in fine chemical production and the strategic sourcing of chemical building blocks.
What are the key market drivers leading to the rise in the adoption of Pharmaceutical IntermediatesMarket Industry?
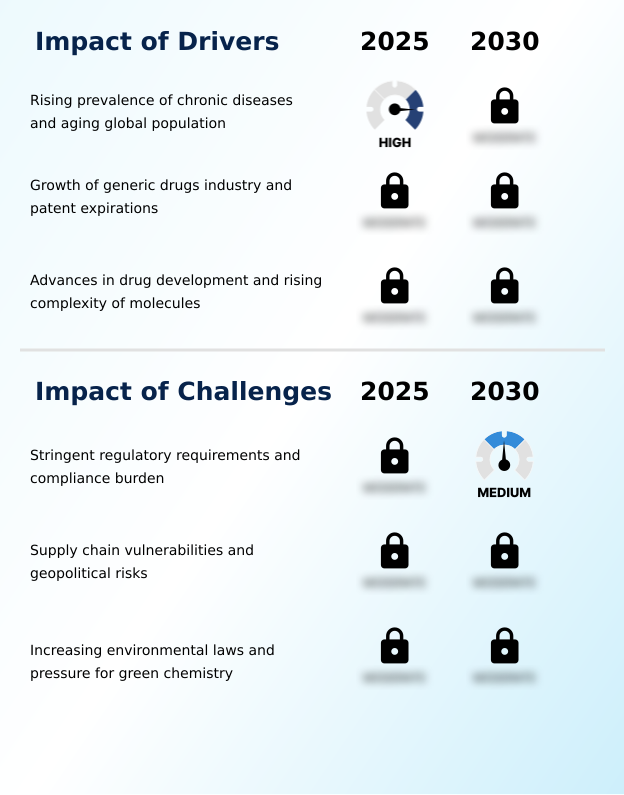
- The rising global prevalence of chronic diseases, combined with a rapidly aging population, creates a sustained and foundational demand for the therapeutic interventions that rely on these intermediates.
- Market expansion is fundamentally propelled by the rising global prevalence of chronic diseases and demographic shifts toward an older population, which ensures a sustained, high-volume need for therapeutics.
- This structural demand for small molecule synthesis is amplified by the burgeoning generic drugs industry.
- The expiration of patents on blockbuster drugs, often referred to as the patent cliff, creates an immediate and massive need for the corresponding active pharmaceutical ingredient production.
- Demand for specific generic intermediates can surge by over 200% in the initial post-patent year.
- Furthermore, relentless innovation in pharmaceutical R&D, particularly the focus on personalized medicine and complex modalities like peptide synthesis and antibody-drug conjugates, is a powerful driver for the high-value segment, requiring a constant supply of novel, sophisticated chiral building blocks for clinical trial materials.
What are the market trends shaping the Pharmaceutical IntermediatesMarket Industry?
- The market is witnessing a pronounced shift toward the development and manufacturing of high-potency and structurally complex intermediates, driven by the demands of targeted therapies.
- A defining trend is the escalating demand for high-potency and structurally complex intermediates, a direct result of the shift toward targeted therapies in oncology and immunology. The synthesis of high-potency APIs (HPAPIs) necessitates a series of specialized intermediates that require contained manufacturing environments to ensure worker safety.
- Firms investing in this niche area have seen a 30% increase in contract value compared to standard intermediate projects. Concurrently, the adoption of advanced manufacturing technologies, particularly flow chemistry technology, is reshaping process development. This approach enhances control over reaction parameters, leading to higher purity and yield.
- CDMOs adopting continuous manufacturing report a 40% reduction in facility footprint and a 15% improvement in batch-to-batch consistency, providing a distinct competitive advantage in a market focused on efficiency and quality.
What challenges does the Pharmaceutical IntermediatesMarket Industry face during its growth?
- Navigating stringent and continuously evolving regulatory requirements, along with the associated compliance burden, presents a significant operational and financial challenge for manufacturers in the industry.
- A formidable challenge is the stringent and complex regulatory landscape governing the pharmaceutical supply chain. Manufacturers of cGMP compliant intermediates face intense scrutiny from bodies like the FDA and EMA, requiring substantial investment in quality systems and meticulous documentation. Adhering to new environmental standards can increase operational costs by up to 20% for manufacturers without established green chemistry principles.
- Another persistent challenge is the inherent vulnerability of a global supply chain heavily concentrated in Asia. Geopolitical tensions and regional disruptions can lead to sudden shortages and price volatility, threatening the production of essential medicines. Recognizing this, regulatory bodies are introducing measures to strengthen the security of medicine supply, adding another layer of compliance complexity for commercial-scale intermediate manufacturing.
Exclusive Technavio Analysis on Customer Landscape
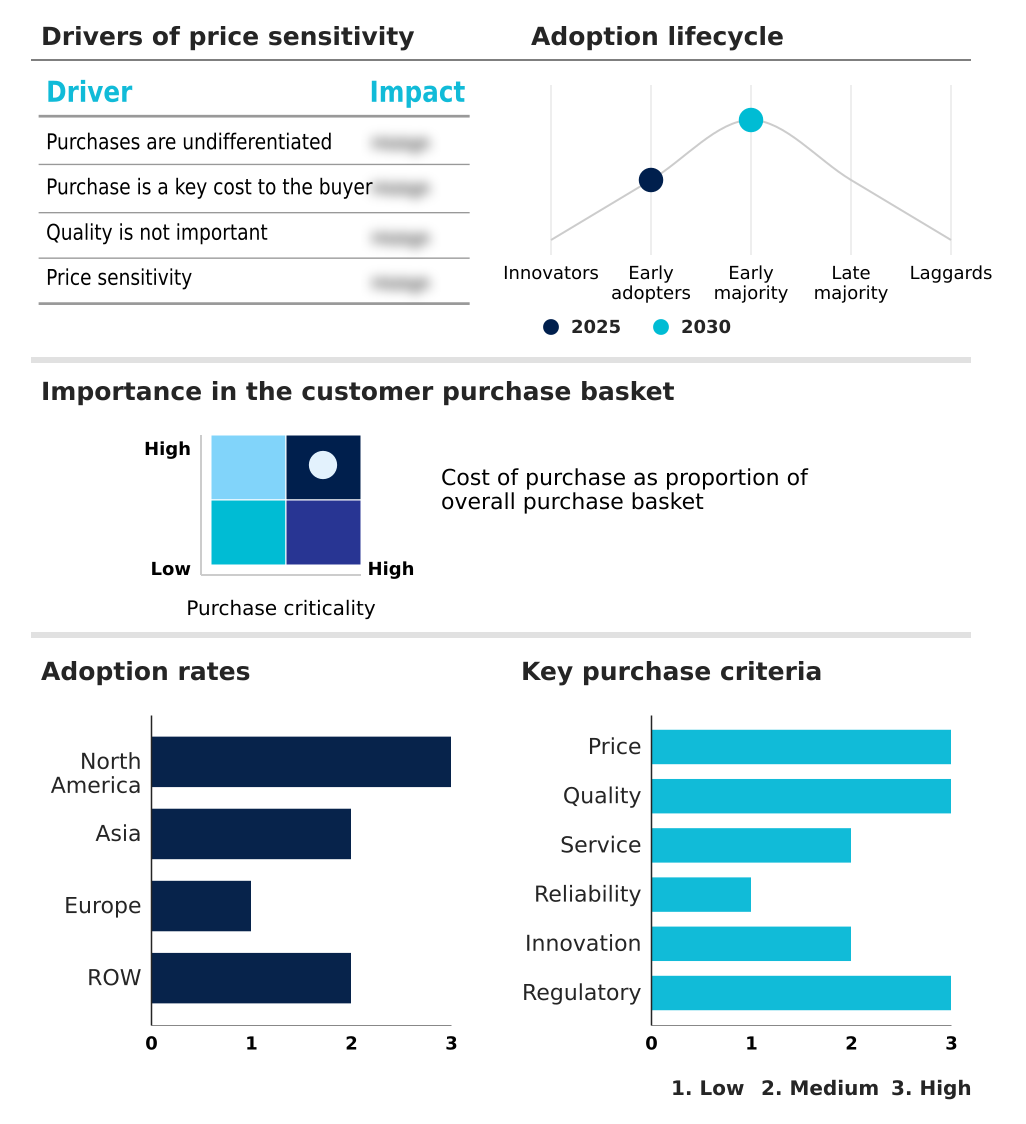
The pharmaceutical intermediates market market forecasting report includes the adoption lifecycle of the market, covering from the innovator’s stage to the laggard’s stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the pharmaceutical intermediates market market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape of Pharmaceutical IntermediatesMarket Industry
Competitive Landscape
Companies are implementing various strategies, such as strategic alliances, pharmaceutical intermediates market market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
Aceto Corp. - Offerings center on custom synthesis and scalable manufacturing of high-purity pharmaceutical intermediates, including advanced chemical building blocks and GMP-compliant starting materials for API production.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Aceto Corp.
- Actylis
- Arkema Group
- Aurobindo Pharma Ltd.
- BASF SE
- Cambrex Corp.
- Carbogen Amcis Ltd.
- EUROAPI S.A.
- Evonik Industries AG
- HIKAL Ltd.
- Hovione S.A.
- Interchem Pvt. Ltd.
- Lianhe Chemical Technology
- Lonza Group Ltd.
- Merck KGaA
- Midas Pharma GmbH
- Thermo Fisher Scientific Inc.
- Vertellus Holdings LLC
- WuXi AppTec Co. Ltd.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Pharmaceutical intermediates market market
- In October 2024, a leading CDMO announced a significant investment to construct a new facility in North America dedicated to the manufacturing of high-potency intermediates and linkers for antibody-drug conjugates (ADCs).
- In January 2025, two prominent pharmaceutical technology firms revealed a strategic partnership to integrate AI-driven platforms into process chemistry, aiming to accelerate synthetic route design and optimization for novel intermediates.
- In March 2025, a major European chemical company launched a new portfolio of bio-based starting materials, developed in alignment with green chemistry principles to reduce the environmental footprint of API synthesis.
- In May 2025, a consortium of top generic drug manufacturers established a joint venture in India to secure the supply chain for key peptide intermediates, responding to the unprecedented demand for GLP-1 agonists.
Dive into Technavio’s robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Pharmaceutical Intermediates Market Market insights. See full methodology.
| Market Scope | |
|---|---|
| Page number | 303 |
| Base year | 2025 |
| Historic period | 2020-2024 |
| Forecast period | 2026-2030 |
| Growth momentum & CAGR | Accelerate at a CAGR of 5.7% |
| Market growth 2026-2030 | USD 13273.6 million |
| Market structure | Fragmented |
| YoY growth 2025-2026(%) | 5.6% |
| Key countries | US, Canada, Mexico, China, India, Japan, South Korea, Indonesia, Thailand, Singapore, Germany, UK, France, The Netherlands, Italy, Spain, Russia, Brazil, Australia, South Africa, UAE, Saudi Arabia and Turkey |
| Competitive landscape | Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
Research Analyst Overview
- The pharmaceutical intermediates market is undergoing a significant transformation, driven by the dual pressures of pharmaceutical innovation and supply chain optimization. The core of this evolution lies in the increasing complexity of molecular architecture in modern therapeutics, particularly small molecule drugs.
- This shift intensifies the demand for advanced intermediates produced through sophisticated synthetic chemistry, including asymmetric catalysis and organometallic chemistry, to achieve precise stereochemistry. As a result, the industry is moving beyond commodity chemicals toward high-value chemical building blocks and fine chemicals.
- Boardroom-level decisions are now heavily focused on capital expenditure for cGMP manufacturing facilities capable of handling high-potency APIs and executing complex process chemistry. For instance, companies that have invested in advanced process development and reaction parameters control have reported a 20% improvement in final product purity.
- The synthesis of these compounds, critical for drug discovery and subsequent drug substance manufacturing, requires deep expertise in medicinal chemistry and a reliable supply of high-purity starting materials. Success hinges on a company's ability to master both the science of API synthesis and the logistics of a global supply chain.
What are the Key Data Covered in this Pharmaceutical Intermediates Market Market Research and Growth Report?
-
What is the expected growth of the Pharmaceutical Intermediates Market Market between 2026 and 2030?
-
USD 13.27 billion, at a CAGR of 5.7%
-
-
What segmentation does the market report cover?
-
The report is segmented by Type (Aromatic intermediates, Heterocyclic intermediates, Chiral intermediates, Functional group intermediates, and Peptide intermediates), Application (Anti-inflammatory drugs, Analgesics, Cardiovascular drugs, Anti Cancer drugs, and Others), End-user (Biotech and Pharma Companies, CMOs and CROs, and Research Laboratory) and Geography (North America, Asia, Europe, Rest of World (ROW))
-
-
Which regions are analyzed in the report?
-
North America, Asia, Europe and Rest of World (ROW)
-
-
What are the key growth drivers and market challenges?
-
Rising prevalence of chronic diseases and aging global population, Stringent regulatory requirements and compliance burden
-
-
Who are the major players in the Pharmaceutical Intermediates Market Market?
-
Aceto Corp., Actylis, Arkema Group, Aurobindo Pharma Ltd., BASF SE, Cambrex Corp., Carbogen Amcis Ltd., EUROAPI S.A., Evonik Industries AG, HIKAL Ltd., Hovione S.A., Interchem Pvt. Ltd., Lianhe Chemical Technology, Lonza Group Ltd., Merck KGaA, Midas Pharma GmbH, Thermo Fisher Scientific Inc., Vertellus Holdings LLC and WuXi AppTec Co. Ltd.
-
Market Research Insights
- The market's momentum is shaped by the strategic decisions of biotech and pharma companies. The trend of outsourcing intermediate production to a contract development and manufacturing organization is accelerating, as it allows innovators to avoid significant capital expenditure and access specialized technologies.
- CDMOs implementing flow chemistry technology have demonstrated the ability to reduce reaction times by over 25% and improve yield consistency compared to traditional batch methods. This efficiency is critical for managing the supply of pharmaceutical raw materials and ensuring supply chain resilience.
- Furthermore, strategic partnerships for custom intermediate synthesis are proving vital for drug substance development, with collaborative projects showing a 15% higher success rate in progressing from preclinical to Phase I trials by leveraging expert scale-up synthesis services and optimized API process optimization.
We can help! Our analysts can customize this pharmaceutical intermediates market market research report to meet your requirements.