Generic Drugs Market Size 2025-2029
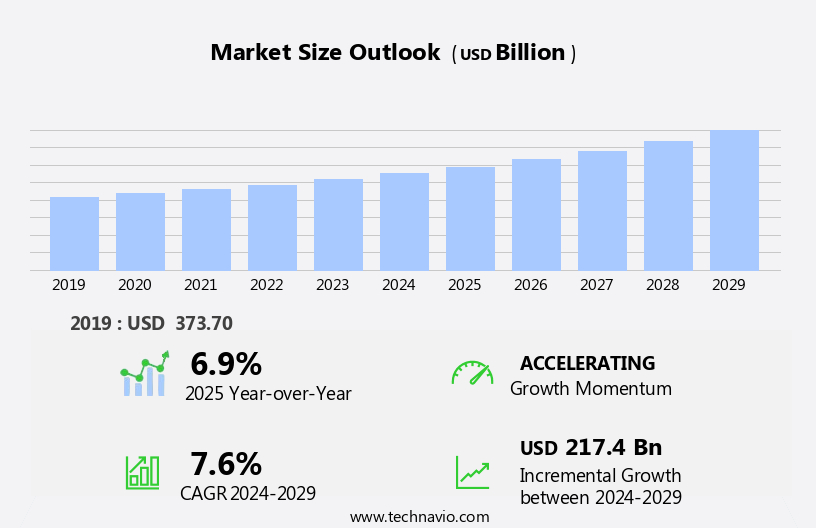
The generic drugs market size is forecast to increase by USD 217.4 billion, at a CAGR of 7.6% between 2024 and 2029.
- The market is driven by the increasing demand for low-cost alternatives to branded medicines. This trend is particularly pronounced in developing economies where affordability is a significant factor in healthcare access. However, the market faces challenges, including the advent of Robotic Process Automation (RPA) in the pharmaceutical industry, which could potentially reduce the cost advantage of generic drugs. Additionally, rising credibility issues related to generic drugs pose a significant challenge, as consumers and healthcare providers increasingly demand assurance of quality and safety. Companies in the market must navigate these challenges by focusing on ensuring the highest standards of quality and safety, while also leveraging technological advancements to maintain cost competitiveness.
- Strategic partnerships, mergers and acquisitions, and collaborations could also provide opportunities for market expansion and growth. Overall, the market presents both opportunities and challenges, requiring a strategic approach from market participants to capitalize on the former and mitigate the latter.
What will be the Size of the Generic Drugs Market during the forecast period?
Explore in-depth regional segment analysis with market size data - historical 2019-2023 and forecasts 2025-2029 - in the full report.
Request Free Sample
The market continues to evolve, shaped by various factors that impact its dynamics. Patient education plays a crucial role in ensuring the effective utilization of medications, with dosage forms varying from capsules to tablets and liquids. Clinical trials and pharmacokinetic studies contribute to regulatory approval, ensuring therapeutic equivalence and drug safety. Pharmaceutical manufacturing involves GMP compliance and stability testing to maintain quality, while patent expiration triggers increased competition and price reductions. Drug interactions necessitate diligent monitoring and education, with regulatory bodies such as the EMA and FDA overseeing approvals. Value-based healthcare and market access strategies prioritize cost-effectiveness analysis, influencing pricing and distribution channels.
Online pharmacies and retail pharmacies adapt to evolving market trends, offering convenience and accessibility. Intellectual property rights and pharmacy dispensing regulations shape the competitive landscape. Bioequivalence studies and drug utilization reviews facilitate generic substitution, while pharmacy dispensing and capsule filling processes ensure accurate and efficient prescription fulfillment. The continuous unfolding of market activities necessitates ongoing attention to drug safety, regulatory compliance, and pricing strategies.
How is this Generic Drugs Industry segmented?
The generic drugs industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD billion" for the period 2025-2029, as well as historical data from 2019-2023 for the following segments.
- Route Of Administration
- Oral
- Injectables
- Topical
- Inhalers
- Type
- Small-molecule generics
- Biosimilars
- Application
- Anti-infectives
- CNS
- Others
- Geography
- North America
- US
- Canada
- Europe
- France
- Germany
- Italy
- UK
- APAC
- China
- India
- Japan
- South America
- Brazil
- Rest of World (ROW)
- North America
.
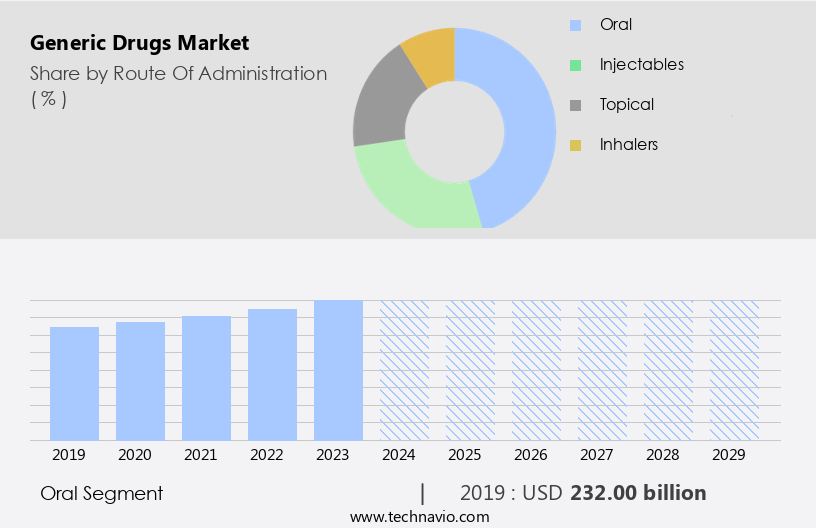
By Route Of Administration Insights
The oral segment is estimated to witness significant growth during the forecast period
The oral route of administration is the most commonly used. Oral administration is the ingestion of a drug through the mouth into the gastrointestinal tract. The oral drug delivery system market segment includes drugs that can be administered in the form of capsules, tablets, syrups, solutions, and suspensions. The growth of the segment is accelerating at a moderate pace due to the ease of usage and an increasing number of companies investing in research and development for oral treatment. The advantages provided by the oral route, such as better availability, rapid drug delivery, and high efficacy, are further expected to propel the growth of the oral segment, which, in turn, will drive the growth of the market during the forecast period.
The Oral segment was valued at USD 232.00 billion in 2019 and showed a gradual increase during the forecast period.
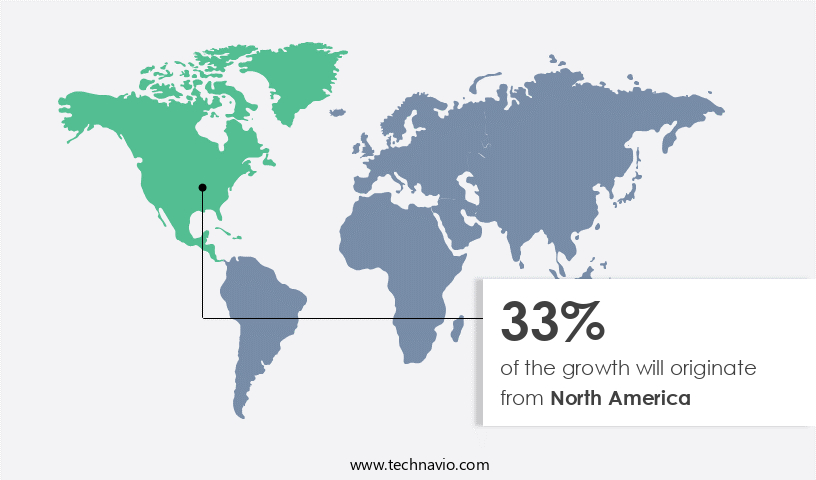
Regional Analysis
North America is estimated to contribute 33% to the growth of the global market during the forecast period. Technavio's analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
The market in North America is experiencing significant growth, with the United States and Canada being key contributors. Major pharmaceutical companies, including Merck (Merck and Co. Inc.), Pfizer (Pfizer Inc.), and Amgen (Amgen Inc.), based in the US, are driving market expansion through the provision of diverse, highly differentiated products. These offerings influence both healthcare professionals and patients, leading to increased adoption. Chronic diseases, such as diabetes and cancer, are on the rise in North America due to lifestyle factors and increased consumption of alcohol and tobacco. In Canada, the demand for biosimilars is increasing due to their therapeutic benefits and cost savings, particularly in the treatment of breast cancer.
Regulatory approval processes, including those by the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA), ensure the safety and efficacy of generic drugs. Pharmaceutical manufacturing companies conduct bioequivalence studies, pharmacokinetic studies, and clinical trials to prove therapeutic equivalence and gain approval. Intellectual property rights and drug utilization reviews are crucial in the distribution and dispensing of generic drugs through various channels, including retail pharmacies, online pharmacies, and wholesale distributors. Quality control and stability testing are essential to maintain drug safety and efficacy throughout the supply chain. Pricing strategies and cost-effectiveness analysis play a significant role in market access and patient adherence.
Drug interactions and drug safety are ongoing concerns, requiring continuous monitoring and education for patients and healthcare professionals.
Market Dynamics
Our researchers analyzed the data with 2024 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
What are the key market drivers leading to the rise in the adoption of Generic Drugs Industry?
- The market is driven primarily by the availability and demand for cost-effective alternatives to branded medications.
- Generic drugs offer significant cost savings for businesses and consumers, as they are priced approximately 85% lower than their brand-name counterparts. This cost difference arises due to the absence of the need for generic drug applicants to repeat extensive animal and clinical studies for safety and effectiveness demonstration, which brand-name drug companies have already conducted. For instance, Novartis AG's Lopressor, a selective beta 1-adrenoreceptor blocking agent, is used for treating various cardiovascular conditions. Metoprolol, the generic version of Lopressor, serves the same therapeutic purpose. The approval process for generic drugs involves various stages, including drug utilization review, GMP compliance, and bioequivalence studies, ensuring the generic drug's safety and efficacy.
- Online pharmacies have emerged as a popular distribution channel for generic drugs, providing convenience and accessibility. Intellectual property rights, however, play a crucial role in the market dynamics, as generic drug companies must secure regulatory approvals, such as EMA approval, before entering the market. Capsule filling and other pharmacy dispensing processes ensure the final product's quality before it reaches the consumer. Overall, the market continues to grow, offering substantial cost savings while maintaining regulatory compliance and therapeutic effectiveness.
What are the market trends shaping the Generic Drugs Industry?
- The adoption of Robotic Process Automation (RPA) is currently a significant market trend. This technology is increasingly being implemented by businesses to streamline operations and improve efficiency.
- Pharmaceutical manufacturing companies are integrating robotic process automation (RPA) technology into their research and development (R&D) and manufacturing processes. RPA utilizes software with AI and machine learning capabilities to manage high-volume, repetitive tasks. This technology allows software to log into applications, enter data, execute calculations, complete tasks, and log out, enhancing regulatory compliance and reducing manual labor costs. Clinical trials are a crucial aspect of bringing new drugs to market. Patient education is essential during this phase to ensure proper drug usage and minimize potential drug interactions. Pharmaceutical companies are investing in RPA to streamline clinical trial processes, from data entry to reporting.
- Upon patent expiration, pharmaceutical companies can produce generic versions of drugs, which undergo rigorous testing to ensure drug safety and efficacy. Tablet compression and liquid formulation are common methods for manufacturing generic drugs. RPA can assist in managing the complex process of generic substitution, ensuring accuracy and efficiency. In conclusion, RPA technology is transforming the pharmaceutical industry by automating repetitive tasks, improving compliance, and reducing costs. Companies continue to invest in this technology for R&D, manufacturing, and generic drug production, ensuring the delivery of safe and effective medications to patients. Recent research emphasizes the importance of RPA in the pharmaceutical sector, with Pfizer and Teva Pharmaceutical being notable adopters.
What challenges does the Generic Drugs Industry face during its growth?
- The escalating concerns regarding the credibility of generic drugs pose a significant challenge to the expansion of the pharmaceutical industry.
- The market faces challenges in establishing credibility due to safety and quality concerns. The prevalence of counterfeit generic drugs, particularly in developing countries, and the rising number of substandard products being withdrawn from the market contribute to this issue. For instance, Pfizer had to recall nearly one million packs of birth control pills due to miscounted and incorrectly ordered inert and active pills in their blister packs. This distrust extends to the US market, where fewer than 40% of patients prefer generic drugs over branded formulations due to perceived safety and quality concerns.
- To mitigate these issues, it's crucial for stakeholders to prioritize stability testing, cost-effectiveness analysis, drug formulation, supply chain management, quality control, and therapeutic equivalence. Ensuring these factors are addressed effectively can help restore confidence in the market and promote their adoption among patients.
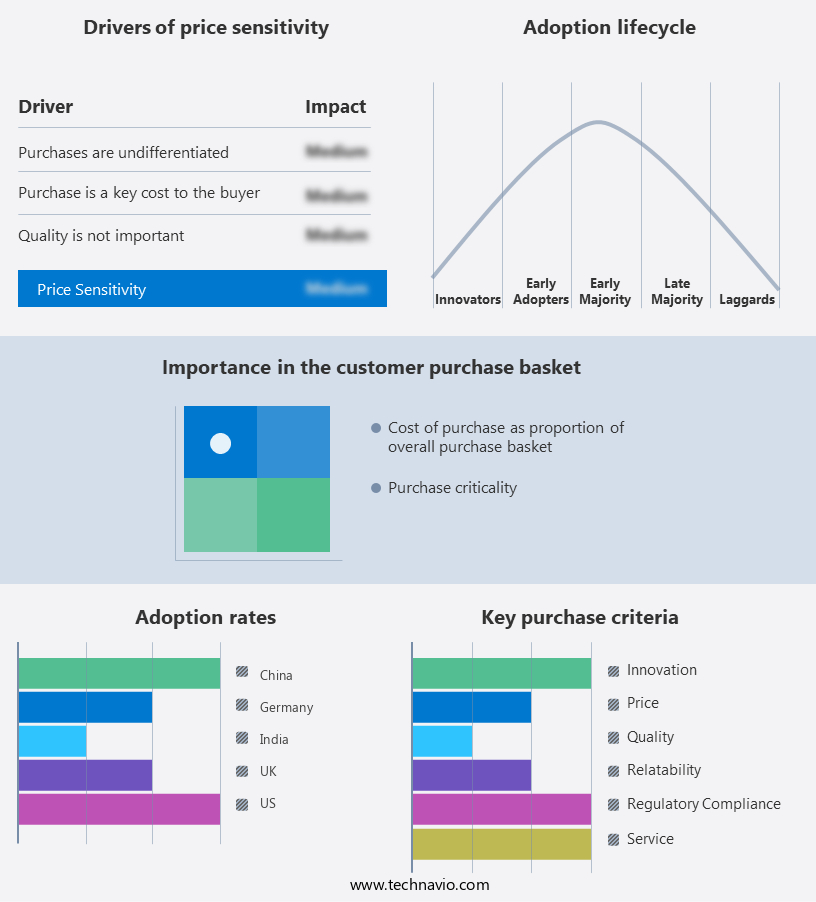
Exclusive Customer Landscape
The generic drugs market forecasting report includes the adoption lifecycle of the market, covering from the innovator's stage to the laggard's stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the generic drugs market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape
Key Companies & Market Insights
Companies are implementing various strategies, such as strategic alliances, generic drugs market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
Amgen Inc. - This company specializes in the distribution of various FDA-approved generic medications, including AIMIVIG for migraine prevention, CORLANOR for heart failure treatment, EPogen for anemia management, and XGEVA for bone health preservation.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Amgen Inc.
- Aurobindo Pharma Ltd.
- Baxter International Inc.
- Biocon Ltd.
- Cadila Pharmaceuticals Ltd.
- Cipla Inc.
- Dr Reddys Laboratories Ltd.
- Eli Lilly and Co.
- Fresenius SE and Co. KGaA
- GlaxoSmithKline Plc
- Lupin Ltd.
- Merck and Co. Inc.
- Novartis AG
- Pfizer Inc.
- Sanofi SA
- Sun Pharmaceutical Industries Ltd.
- Teva Pharmaceutical Industries Ltd.
- Viatris Inc.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Generic Drugs Market
- In March 2024, the U.S. Food and Drug Administration (FDA) approved Mylan NV's generic version of Revlimid, a cancer drug previously sold as a branded product by Celgene Corporation. This approval marked a significant expansion for Mylan in the oncology drugs market (FDA, 2024).
- In July 2024, Teva Pharmaceutical Industries Ltd. and Sandoz, a Novartis AG subsidiary, announced a strategic collaboration to co-develop and commercialize 30 generic products in the U.S. And Europe. This partnership aimed to enhance their combined market presence and reduce R&D costs (Teva, 2024).
- In December 2024, Hikma Pharmaceuticals PLC received approval from the European Medicines Agency (EMA) to manufacture and sell a generic version of Gilead Sciences Inc.'s HIV medication, Tenofovir Disoproxil Fumarate/Emtricitabine. This approval granted Hikma access to the European market, expanding its global footprint (Hikma, 2024).
- In February 2025, Dr. Reddy's Laboratories Ltd. and Emcure Pharmaceuticals Ltd. Entered into a definitive agreement to merge their generic injectables business. This merger aimed to create a leading player in the global injectables market, combining their expertise and resources (Dr. Reddy's, 2025).
Research Analyst Overview
- In the dynamic pharmaceutical landscape, the market for generic drugs continues to gain momentum, driven by advancements in biopharmaceutical manufacturing and pharmaceutical chemistry. Process validation and quality assurance play crucial roles in ensuring the consistency and safety of generic drugs. Pharmacokinetic modeling and statistical analysis help predict drug behavior in patients, enhancing patient satisfaction and medication safety. Reverse engineering and targeted drug delivery systems enable the development of high-quality generic drugs, challenging branded counterparts in areas like controlled release and prescription drug monitoring.
- Healthcare policy and health economics significantly impact the generic drug market, influencing brand switching and drug information dissemination. Pharmaceutical engineering, clinical pharmacology, and clinical trial design are essential components of the generic drug development process, ensuring drug metabolism, excretion, and health outcomes research align with regulatory standards. Analytical methods and drug discovery continue to advance, offering opportunities for innovation in the off-patent drugs sector.
Dive into Technavio's robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Generic Drugs Market insights. See full methodology.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
221 |
|
Base year |
2024 |
|
Historic period |
2019-2023 |
|
Forecast period |
2025-2029 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 7.6% |
|
Market growth 2025-2029 |
USD 217.4 billion |
|
Market structure |
Fragmented |
|
YoY growth 2024-2025(%) |
6.9 |
|
Key countries |
US, China, Japan, Germany, India, UK, France, Brazil, Canada, and Italy |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
What are the Key Data Covered in this Generic Drugs Market Research and Growth Report?
- CAGR of the Generic Drugs industry during the forecast period
- Detailed information on factors that will drive the growth and forecasting between 2025 and 2029
- Precise estimation of the size of the market and its contribution of the industry in focus to the parent market
- Accurate predictions about upcoming growth and trends and changes in consumer behaviour
- Growth of the market across North America, Europe, Asia, and Rest of World (ROW)
- Thorough analysis of the market's competitive landscape and detailed information about companies
- Comprehensive analysis of factors that will challenge the generic drugs market growth of industry companies
We can help! Our analysts can customize this generic drugs market research report to meet your requirements.