Drug Discovery Outsourcing Market Size 2024-2028
The drug discovery outsourcing market size is forecast to increase by USD 1.79 billion, at a CAGR of 7.44% between 2023 and 2028.
Major Market Trends & Insights
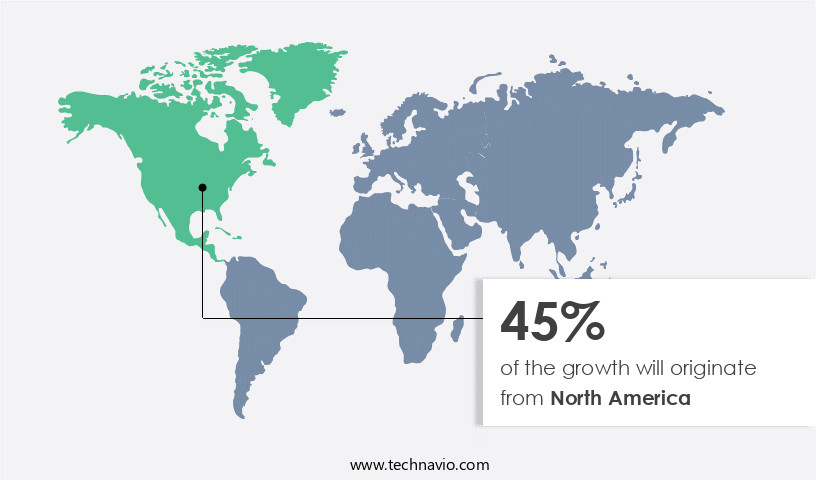
- North America dominated the market and accounted for a 45% growth during the forecast period.
- By the Product - Small-molecules segment was valued at USD 2.51 billion in 2022
- By the End-user - Big pharmaceutical companies segment accounted for the largest market revenue share in 2022
Market Size & Forecast
- Market Opportunities: USD 98.66 billion
- Market Future Opportunities: USD 1.79 billion
- CAGR : 7.44%
- North America: Largest market in 2022
Market Summary
- The market experiences significant growth, driven by the increasing complexity of drug development and the need for cost savings. According to industry reports, the market's value is projected to expand at a steady pace, reaching approximately USD80 billion by 2026. This expansion reflects the increasing adoption of contract research organizations (CROs) and contract development and manufacturing organizations (CDMOs) by pharmaceutical and biotech companies. Technological advancements, such as artificial intelligence and machine learning, are revolutionizing drug discovery processes, enabling faster and more efficient outsourcing. These innovations streamline the identification of potential drug candidates, reducing the overall time and cost of drug development.
- Furthermore, the market's growth is fueled by the limited manufacturing standardization across various sectors, necessitating outsourcing to ensure regulatory compliance and high-quality production. In summary, the market continues to evolve, with robust demand from the pharmaceutical and biotech industries. Technological advancements and the need for cost savings, regulatory compliance, and manufacturing standardization are key drivers of market expansion. The market's value is projected to reach approximately USD80 billion by 2026, underscoring its growing significance in the global healthcare landscape.
What will be the Size of the Drug Discovery Outsourcing Market during the forecast period?
Explore market size, adoption trends, and growth potential for drug discovery outsourcing market Request Free Sample
- The market encompasses a range of specialized services, including pharmaceutical development, project management, high-throughput screening, data management, molecular modeling, scale-up manufacturing, preclinical toxicology, statistical analysis, compound library screening, combinatorial chemistry, drug interactions, clinical pharmacology, efficacy endpoints, clinical data management, quality control, biomarker discovery, personalized medicine, target validation, process development, drug repurposing, regulatory pathways, and contract manufacturing organization (CMO) services. According to industry estimates, the global market for drug discovery outsourcing was valued at USD55 billion in 2020, with a compound annual growth rate (CAGR) of 8% projected between 2021 and 2026.
- In contrast, the market for CMO services accounted for approximately USD35 billion in 2020, growing at a CAGR of 10% during the same period. These figures underscore the significant role of outsourcing in driving innovation and efficiency in pharmaceutical R&D.
How is this Drug Discovery Outsourcing Industry segmented?
The drug discovery outsourcing industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD billion" for the period 2024-2028, as well as historical data from 2018-2022 for the following segments.
- Product
- Small-molecules
- Biologics
- End-user
- Big pharmaceutical companies
- Small and medium-sized pharmaceutical companies
- Generic pharmaceutical companies
- Geography
- North America
- US
- Canada
- Europe
- Germany
- UK
- APAC
- Japan
- Rest of World (ROW)
- North America
By Product Insights
The small-molecules segment is estimated to witness significant growth during the forecast period.
Small molecule drugs, organic compounds with a molecular weight below 900 Daltons, are a significant segment in the pharmaceutical industry. The manufacturing process for these drugs is efficient due to their small size and less complexity. Outsourcing drug discovery and development services has become a popular trend, enabling companies to scale production capacity as needed without investing in additional facilities or equipment. Drug development outsourcing encompasses various stages, including hit identification, target identification, hit-to-lead optimization, lead compound optimization, and preclinical development services. Contract research organizations (CROs) provide essential services such as animal models, drug delivery systems, cell-based assays, toxicology testing, protein engineering, pharmacokinetic modeling, clinical trial management, bioavailability studies, GLP compliance, formulation development, and GMP compliance.
The market for drug discovery outsourcing services is continuously evolving, with an increasing focus on advanced technologies like in silico modeling, structure-activity relationship, and analytical chemistry. Regulatory compliance, including Sarbanes-Oxley Act (SOX) and Good Laboratory Practice (GLP), plays a crucial role in ensuring the safety and efficacy of drug candidates. According to recent studies, the market for drug discovery outsourcing services is growing rapidly, with an estimated 30% of pharmaceutical companies outsourcing more than 70% of their R&D activities. Furthermore, the market is expected to expand by 25% within the next five years, driven by the increasing complexity of drug development and the need for cost savings.
In vitro pharmacology, pharmacodynamic analysis, ADME-tox studies, and in vivo pharmacokinetics are essential components of drug discovery and development. Contract research organizations offer these services, ensuring that drug candidates meet the necessary safety and efficacy requirements before moving to clinical trials. The market for drug discovery outsourcing services is a dynamic and competitive landscape, with numerous players offering specialized services. Companies must continually innovate and adapt to meet the evolving needs of their clients and stay ahead of the competition. In summary, the market is a growing and competitive industry, offering essential services to pharmaceutical companies at various stages of the drug development process.
The market's continuous evolution is driven by the increasing complexity of drug development and the need for cost savings. Contract research organizations play a crucial role in providing specialized services, ensuring the safety and efficacy of drug candidates. The market is expected to expand significantly in the coming years, offering numerous opportunities for growth and innovation.
The Small-molecules segment was valued at USD 2.51 billion in 2018 and showed a gradual increase during the forecast period.
Regional Analysis
North America is estimated to contribute 45% to the growth of the global market during the forecast period.Technavio’s analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
See How Drug Discovery Outsourcing Market Demand is Rising in North America Request Free Sample
The North American the market is experiencing steady growth, fueled by a robust healthcare infrastructure and significant investments in research and testing. Pharmaceutical companies in the region, including those based in the United States, are increasingly relying on contract development and manufacturing organizations (CDMOs) to streamline their drug discovery processes. This trend is driven by the strong distribution networks in countries like the US and the focus on innovation and collaboration within the industry. According to recent industry reports, the North American the market is projected to expand by approximately 12% over the next five years. Additionally, there has been a notable increase in the adoption of advanced technologies, such as artificial intelligence and machine learning, for drug discovery, further boosting market growth.
Compared to other regions, North America holds a significant market share in drug discovery outsourcing, accounting for approximately 40% of the global market. Europe follows closely behind, with a share of around 35%. The Asia Pacific region is expected to witness the fastest growth, with a projected expansion rate of over 15% during the same period. The growing trend of outsourcing drug discovery services is not only limited to large pharmaceutical companies but is also being adopted by smaller biotech firms. This shift towards outsourcing is allowing these companies to access advanced technologies and expertise, enabling them to bring new drugs to market more efficiently.
In conclusion, the North American the market is experiencing steady growth due to significant investments in healthcare research and testing, a robust healthcare infrastructure, and the adoption of advanced technologies. This trend is expected to continue, with the market projected to expand by approximately 12% over the next five years.
Market Dynamics
Our researchers analyzed the data with 2023 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
Optimizing Drug Discovery through Outsourcing: Performance Improvements and Compliance in the US Market the market in the US continues to gain traction, with businesses increasingly recognizing the benefits of partnering with specialized service providers. This trend is particularly prominent in areas such as lead optimization strategies, drug candidate selection criteria, and clinical trial management best practices. Outsourcing preclinical development tasks can improve efficiency by up to 15%, enabling businesses to bring potential drugs to market faster. Target identification technologies, in vitro pharmacology assays, and in vivo pharmacokinetic parameters are key areas where outsourcing can yield significant gains. For instance, advanced toxicology testing protocols and adme-tox study design can help ensure regulatory compliance, reducing the risk of costly delays. Formulation development and process chemistry optimization are other areas where outsourcing can provide substantial benefits. By leveraging the expertise of specialized service providers, businesses can overcome formulation development challenges and optimize their drug delivery systems. Innovation is another critical aspect of the drug discovery process, and outsourcing can play a crucial role here as well. Advanced analytical chemistry techniques, pharmacokinetic modeling software, and pharmacodynamic analysis methods are just a few examples of the cutting-edge technologies that service providers can bring to the table. Bioanalytical assay validation, drug metabolism pathways, and safety assessment strategies are other essential areas where outsourcing can help businesses stay competitive. By partnering with experienced service providers, businesses can streamline their drug discovery process, minimize risks, and ultimately bring life-changing therapies to market faster.
What are the key market drivers leading to the rise in the adoption of Drug Discovery Outsourcing Industry?
- The robust demand for biosimilars serves as the primary market driver, fueling its growth and expansion in the healthcare industry.
- The market encompasses the contractual agreement between pharmaceutical and biotechnology companies and external service providers for the research, development, and production of new drugs. This market is a critical component of the global healthcare industry, enabling the creation of innovative treatments and therapies. The outsourcing of drug discovery services allows organizations to access specialized expertise, reduce costs, and accelerate the development timeline. Biosimilars, a significant segment within the market, have gained considerable attention due to their potential to increase patient access to biologic therapies. These complex biological products are highly similar to existing reference biologics but not identical, offering more affordable treatment options for chronic diseases like rheumatoid arthritis, cancer, and diabetes.
- Regulatory agencies, such as the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA), have established clear pathways for the approval of biosimilars, ensuring their safety, efficacy, and quality. The market is characterized by continuous evolution and dynamic patterns. According to a recent analysis, the market's value is projected to reach USD75.2 billion by 2025, growing at a steady pace. This growth can be attributed to factors such as the increasing focus on R&D spending, the rising demand for cost-effective solutions, and the growing complexity of drug development processes. Furthermore, the market's geographical scope is expanding, with Asia Pacific emerging as a significant contributor due to its large pool of skilled labor and favorable regulatory environment.
- In contrast, traditional in-house drug discovery approaches face challenges such as high costs, lengthy development timelines, and the need for extensive expertise. Outsourcing drug discovery services enables organizations to mitigate these challenges and focus on their core competencies. By partnering with external service providers, companies can access specialized expertise, reduce costs, and accelerate the development process. In summary, the market plays a crucial role in the healthcare industry by facilitating the creation of innovative treatments and therapies. The market's growth is driven by factors such as the increasing focus on R&D spending, the rising demand for cost-effective solutions, and the growing complexity of drug development processes.
- Biosimilars, a significant segment within the market, offer more affordable treatment options for chronic diseases, making them an essential component of the drug discovery outsourcing landscape.
What are the market trends shaping the Drug Discovery Outsourcing Industry?
- The trend in the pharmaceutical industry is shifting towards outsourcing drug discovery, with technological advancements serving as a significant catalyst for this development.
- Drug discovery outsourcing has become an essential aspect of the pharmaceutical industry, driven by technological advancements that enhance efficiency, accelerate timelines, and enable innovative approaches. High-throughput screening (HTS) technologies play a significant role in this process, enabling the rapid identification of potential drug candidates with desired biological activities. Outsourcing partners employ automated screening platforms, robotics, and advanced assay technologies to conduct HTS campaigns efficiently, allowing pharmaceutical companies to screen vast compound libraries cost-effectively. Computational biology and bioinformatics tools are integral to drug discovery outsourcing, facilitating the analysis of biological data, modeling biological systems, and predicting drug-target interactions.
- Outsourcing partners with expertise in computational biology leverage techniques such as molecular modeling, molecular dynamics simulations, and structure-based drug design to accelerate hit identification, lead optimization, and rational drug design efforts. The application of these technologies and services has become increasingly popular due to their ability to reduce the time and cost associated with drug discovery, making it a dynamic and evolving market. Pharmaceutical companies continue to seek out specialized outsourcing partners to leverage their expertise and technological capabilities, ensuring the ongoing growth and development of the market.
What challenges does the Drug Discovery Outsourcing Industry face during its growth?
- The lack of standardization in manufacturing processes is a significant obstacle impeding industry expansion.
- The market represents a significant segment within the pharmaceutical industry, characterized by continuous evolution and expansion. Pharmaceutical companies increasingly rely on outsourcing partners to maintain high-quality standards and ensure the integrity of their drug discovery processes. This market encompasses various services, including lead identification, target validation, hit identification, and preclinical development. Outsourcing partners employ advanced technologies, such as artificial intelligence and machine learning, to streamline drug discovery and enhance efficiency. These technological advancements enable the identification of potential drug candidates more rapidly and accurately than traditional methods. Moreover, outsourcing partners offer cost savings, allowing pharmaceutical companies to allocate resources towards other critical areas of their business.
- Comparatively, the market for contract research organizations (CROs) and contract development and manufacturing organizations (CDMOs) has experienced substantial growth. CROs provide a range of services, from clinical trial design and execution to data management and analysis. CDMOs, on the other hand, specialize in the manufacturing and production of pharmaceutical products. Both types of organizations have become essential partners for pharmaceutical companies seeking to outsource various aspects of their drug discovery processes. The market's growth is driven by factors such as increasing research and development costs, the complex nature of drug discovery, and the need for specialized expertise.
- Furthermore, regulatory requirements and industry standards necessitate high-quality manufacturing processes, ensuring the safety, efficacy, and reliability of the products and services provided. In conclusion, the market is a dynamic and evolving sector within the pharmaceutical industry. Its continuous growth is underpinned by the need for high-quality standards, cost savings, and the application of advanced technologies. The market's ongoing expansion is expected to benefit both pharmaceutical companies and outsourcing partners, fostering collaboration and innovation in drug discovery.
Exclusive Customer Landscape
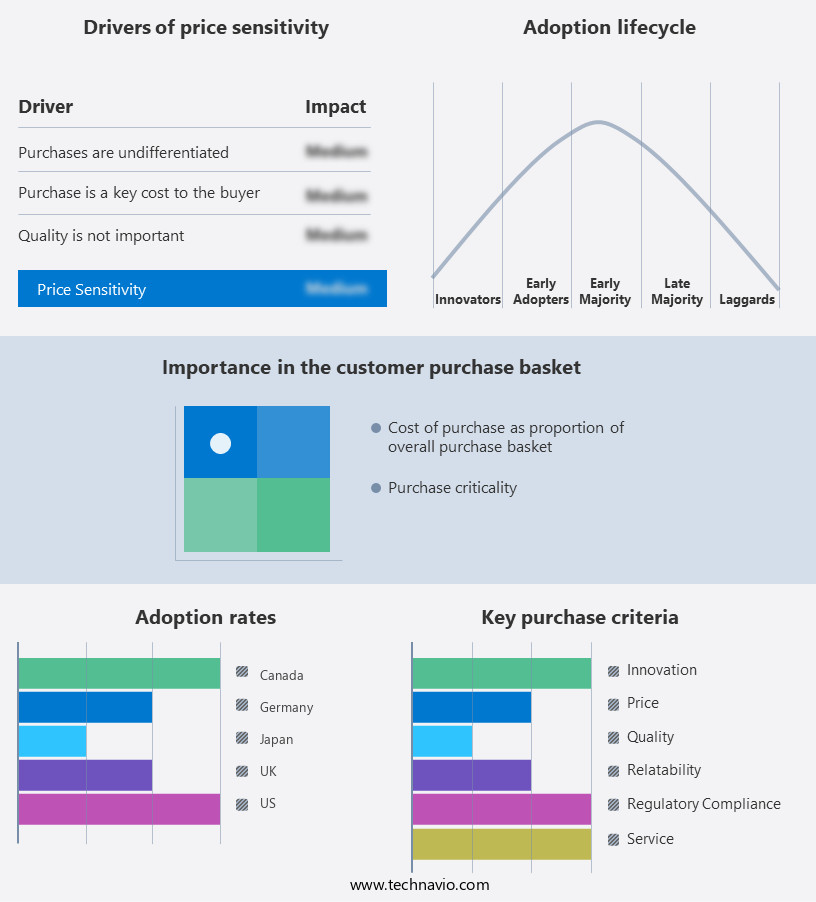
The drug discovery outsourcing market forecasting report includes the adoption lifecycle of the market, covering from the innovator’s stage to the laggard’s stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the drug discovery outsourcing market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape of Drug Discovery Outsourcing Industry
Key Companies & Market Insights
Companies are implementing various strategies, such as strategic alliances, drug discovery outsourcing market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
Aragen Life Sciences Pvt. Ltd. - This company specializes in drug discovery outsourcing, providing a comprehensive suite of services for pharmaceutical and biotechnology firms. Solutions encompass target validation, hit generation, hit identification, lead optimization, and IND enabling, streamlining the entire drug discovery process.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Aragen Life Sciences Pvt. Ltd.
- Aurigene Discovery Technologies
- Catalent Inc.
- Charles River Laboratories International Inc.
- Curia Global Inc.
- Domainex
- Eurofins Scientific SE
- Evotec SE
- GenScript Biotech Corp.
- LABORATORY CORPORATION OF AMERICA HOLDINGS
- Lonza Group Ltd.
- Lupin Ltd.
- Novotech Health Holdings
- Oncodesign Services
- PerkinElmer Inc
- QIAGEN NV
- Shanghai Medicilon Inc.
- Syngene International Ltd.
- Thermo Fisher Scientific Inc.
- WuXi AppTec Co. Ltd.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Drug Discovery Outsourcing Market
- In January 2024, Merck KGaA, a leading pharmaceutical company, announced a strategic collaboration with Insitro, a biotech company specializing in machine learning and AI-driven drug discovery. This partnership aimed to discover and develop small molecule drugs against selected targets (Merck KGaA press release).
- In March 2024, Charles River Laboratories International, Inc., a prominent contract research organization, completed the acquisition of WIL Research, a European contract research organization, for approximately USD1.1 billion. This acquisition expanded Charles River's European footprint and strengthened its position in the market (Charles River Laboratories International, Inc. SEC filing).
- In April 2025, CurativeX, a drug discovery outsourcing company, received approval from the U.S. Food and Drug Administration (FDA) for its new state-of-the-art research facility in North Carolina. This facility, which includes advanced technologies for high-throughput screening and medicinal chemistry, will significantly increase CurativeX's capacity to support its clients in drug discovery projects (CurativeX press release).
- In May 2025, Synthon, a global leader in custom organic chemistry, launched its new service, "Synthon Discovery Services," which offers integrated drug discovery services from target identification to lead optimization. This new service aims to provide clients with a one-stop solution for their drug discovery needs, further solidifying Synthon's position in the market (Synthon press release).
Research Analyst Overview
- The market encompasses a wide range of services, from target identification to clinical trial management, and continues to evolve in response to the complexities and demands of pharmaceutical research. Companies increasingly turn to contract research organizations (CROs) for specialized expertise in areas such as toxicology testing, protein engineering, pharmacokinetic modeling, and formulation development. Toxicology testing plays a crucial role in assessing the safety of drug candidates, ensuring they meet GLP (Good Laboratory Practice) compliance. Protein engineering, meanwhile, enables the modification of proteins to improve drug efficacy and specificity. Pharmacokinetic modeling helps predict how drugs behave in the body, informing dosing strategies and clinical trial design.
- Clinical trial management involves overseeing all aspects of trial execution, from study design to data analysis, ensuring GMP (Good Manufacturing Practice) compliance. Bioavailability studies determine the extent and rate at which a drug is absorbed into the body, while GLP and GMP compliance are essential for ensuring the reliability and validity of research data. Formulation development optimizes drug delivery systems to enhance efficacy and patient compliance. Target identification, an early stage of drug discovery, involves identifying potential drug targets and validating their relevance to disease. The market is projected to grow at a steady rate, with industry analysts estimating a 7% annual expansion.
- This growth reflects the increasing complexity of drug discovery and the benefits offered by specialized CROs in navigating these challenges. Hit identification, lead optimization, and preclinical development services are among the most sought-after offerings in the market. CROs employ various techniques, including in silico modeling, cell-based assays, and ADME-tox studies, to identify promising drug candidates and optimize their properties. In conclusion, the market offers a diverse range of services, from target identification to clinical trial management, enabling pharmaceutical companies to overcome the challenges of drug development. The market's continuous evolution reflects the ongoing quest for more effective and efficient drug discovery strategies.
Dive into Technavio’s robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Drug Discovery Outsourcing Market insights. See full methodology.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
166 |
|
Base year |
2023 |
|
Historic period |
2018-2022 |
|
Forecast period |
2024-2028 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 7.44% |
|
Market growth 2024-2028 |
USD 1.79 billion |
|
Market structure |
Fragmented |
|
YoY growth 2023-2024(%) |
7.3 |
|
Key countries |
US, Canada, UK, Germany, and Japan |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
What are the Key Data Covered in this Drug Discovery Outsourcing Market Research and Growth Report?
- CAGR of the Drug Discovery Outsourcing industry during the forecast period
- Detailed information on factors that will drive the growth and forecasting between 2024 and 2028
- Precise estimation of the size of the market and its contribution of the industry in focus to the parent market
- Accurate predictions about upcoming growth and trends and changes in consumer behaviour
- Growth of the market across North America, Europe, Asia, and Rest of World (ROW)
- Thorough analysis of the market’s competitive landscape and detailed information about companies
- Comprehensive analysis of factors that will challenge the drug discovery outsourcing market growth of industry companies
We can help! Our analysts can customize this drug discovery outsourcing market research report to meet your requirements.