Human Combination Vaccines Market Size 2025-2029
The human combination vaccines market size is forecast to increase by USD 7.72 billion, at a CAGR of 8.8% between 2024 and 2029.
- The market is characterized by significant growth opportunities and challenges. Key drivers include the increasing prevalence of infectious diseases and the growing awareness of the importance of vaccination in preventing them. However, the market also faces challenges, including the rising threat of antiviral drug resistance, which can reduce the effectiveness of some vaccines. Furthermore, the threat from bioterrorism has led to an increased focus on developing combination vaccines that can protect against multiple diseases simultaneously. Consolidation of different immunization schedules is another trend shaping the market, as healthcare providers seek to simplify vaccine administration and improve patient compliance.
- Companies in this market must navigate these challenges while capitalizing on the growing demand for combination vaccines to remain competitive and succeed. Effective strategies may include investing in research and development of new combination vaccines, expanding production capacity, and collaborating with healthcare providers to streamline immunization schedules. By addressing these challenges and leveraging market opportunities, companies can position themselves for long-term success in the market.
What will be the Size of the Human Combination Vaccines Market during the forecast period?
Explore in-depth regional segment analysis with market size data - historical 2019-2023 and forecasts 2025-2029 - in the full report.
Request Free Sample
The market continues to evolve, driven by the ongoing development of new technologies and applications across various sectors. Pneumococcal disease remains a significant focus, with research and innovation centered around pneumococcal conjugate vaccines, inactivated vaccines, and polysaccharide vaccines. The use of viral vectors, needle-free injection, and immunization programs is expanding, enhancing vaccine access and effectiveness. Market dynamics are shaped by factors such as regulatory approvals, cold chain management, and immunization schedules. Pharmaceutical companies are investing in peptide vaccines, t-cell response, and DNA vaccines, aiming to improve vaccine efficacy and healthcare costs. Market access remains a critical concern, with efforts to address vaccine hesitancy and ensure equitable distribution.
The manufacturing process, quality control, and vaccine shelf life are essential considerations, with ongoing research into vaccine delivery systems, recombinant vaccines, and MRNA technology. Health outcomes and healthcare costs are under constant scrutiny, with global health initiatives and vaccination rates playing a crucial role in disease prevention and pandemic preparedness. Vaccine development pipelines are continually evolving, with clinical trials exploring the potential of new adjuvants, vaccine adjuvants, and vaccine effectiveness. The role of intellectual property, disease surveillance, and antibody response in shaping the market landscape is an ongoing discussion. The market's continuous dynamism underscores the importance of staying informed about the latest trends and developments.
How is this Human Combination Vaccines Industry segmented?
The human combination vaccines industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2025-2029, as well as historical data from 2019-2023 for the following segments.
- Type
- Inactivated vaccine
- Live attenuated vaccine
- Channel
- Hospitals
- Retailers
- Online
- Route Of Administration
- Intramuscular
- Subcutaneous
- Oral
- Intradermal
- Nasal spray
- Geography
- North America
- US
- Canada
- Mexico
- Europe
- France
- Germany
- Italy
- UK
- APAC
- China
- India
- Japan
- Rest of World (ROW)
- North America
By Type Insights
The inactivated vaccine segment is estimated to witness significant growth during the forecast period.
The market encompasses a range of vaccine types, including inactivated, live-attenuated, subunit, conjugate, recombinant, peptide, and DNA vaccines. Inactivated vaccines, which account for the largest market share, utilize a process where pathogen particles are destroyed or killed, ensuring they cannot replicate. This segment's dominance is attributed to their better tolerability and fewer complications when combining different antigens. However, producing combination vaccines poses challenges due to potential incompatibilities and interactions among various components. Public awareness campaigns and regulatory approvals play a crucial role in driving the market, with a focus on pandemic preparedness and disease prevention. Vaccine packaging, cold chain management, and storage and stability are essential considerations to maintain vaccine efficacy.
Clinical trials, vaccine delivery systems, and quality control are integral parts of the vaccine development pipeline. Global health initiatives, intellectual property, and disease surveillance are key factors influencing market dynamics. Vaccine hesitancy and healthcare costs are ongoing challenges. Pharmaceutical companies employ various pricing strategies, vaccine adjuvants, and manufacturing processes to enhance vaccine effectiveness and shelf life. MRNA technology, adenovirus vectors, and viral vectors are emerging trends in the market. Needle-free injection systems, t-cell response, and DNA vaccines are also gaining attention. Market access and immunization programs are crucial for ensuring widespread distribution and vaccination rates. The market is a complex ecosystem, requiring robust collaboration among public health, healthcare providers, and regulatory bodies to ensure vaccine safety, efficacy, and affordability.
The vaccine development pipeline is continually evolving, with ongoing research into new technologies and vaccine types to address various diseases and health outcomes. Despite the challenges, the market offers significant opportunities for innovation and growth, with a focus on improving vaccine accessibility, affordability, and efficacy. The combination of scientific advancements, regulatory support, and public awareness campaigns will continue to shape the market landscape.
The Inactivated vaccine segment was valued at USD 9.41 billion in 2019 and showed a gradual increase during the forecast period.
Regional Analysis
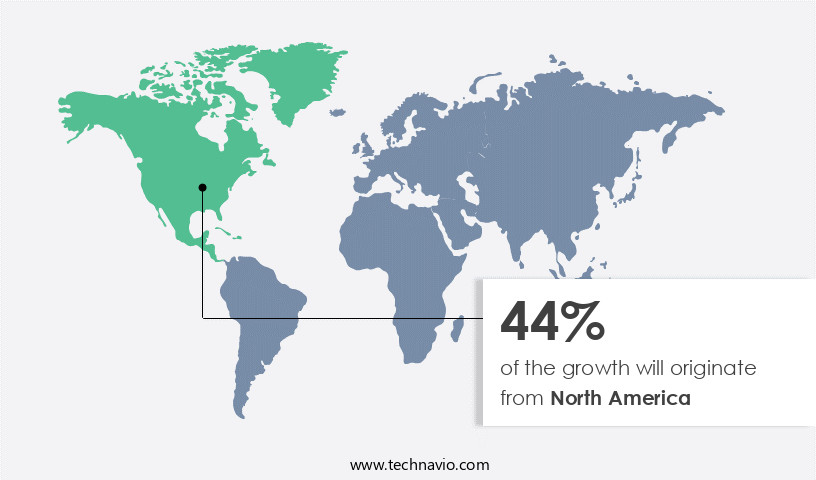
North America is estimated to contribute 48% to the growth of the global market during the forecast period.Technavio’s analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
The market is experiencing significant growth due to the increasing emphasis on disease prevention and pandemic preparedness. Combination vaccines, which contain multiple antigens in a single dose, offer several advantages, including convenience, cost savings, and improved immunization schedules. These vaccines are available in various forms, including inactivated, live-attenuated, subunit, conjugate, recombinant, and mRNA. License agreements between pharmaceutical companies and regulatory bodies play a crucial role in the market's dynamics. For instance, regulatory approvals for new vaccines and vaccine adjuvants can significantly impact market access and pricing strategies. Cold chain management and storage stability are essential considerations for vaccine manufacturers, ensuring vaccine efficacy and safety.
Public health initiatives and healthcare providers are key players in promoting vaccination programs and ensuring vaccine distribution networks are efficient. The market for human combination vaccines is expected to grow, driven by ongoing clinical trials, vaccine development pipelines, and global health initiatives. However, challenges such as vaccine hesitancy, adverse events, and disease surveillance remain critical concerns. Inactivated vaccines, such as those for polio and measles, have a long history of success, while newer technologies, like mRNA and adenovirus vectors, are gaining popularity. The manufacturing process for these vaccines involves complex quality control measures to ensure antibody response and vaccine shelf life.
Despite the challenges, the market continues to evolve, with innovations in vaccine delivery systems, needle-free injection technologies, and t-cell response-enhancing peptide vaccines. The market is also exploring new opportunities in DNA vaccines and protein-based vaccines. As the market grows, healthcare outcomes and healthcare costs are becoming increasingly important considerations. In North America, the market is the largest, driven by strict regulatory norms and ongoing efforts to promote vaccination programs. However, the market is expected to grow at a slower rate due to the decreasing prevalence of infectious diseases, such as polio, in the region. Nonetheless, the market's future looks promising, with ongoing research and development efforts and a growing focus on disease prevention and pandemic preparedness.
Market Dynamics
Our researchers analyzed the data with 2024 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
The market represents a significant segment of the global pharmaceutical industry, focusing on the development, production, and distribution of vaccines containing multiple antigens. These vaccines offer enhanced protection against multiple diseases with a single administration, revolutionizing public health strategies. Key players in this market invest heavily in research and development, leveraging advanced technologies such as adjuvants, nanoparticles, and molecular biology. Combination vaccines address pressing health concerns, including measles, mumps, rubella, polio, and hepatitis A and B. They also target emerging diseases, such as dengue fever and influenza, ensuring broad immunization and disease prevention. This market is driven by increasing global awareness of vaccine importance, government initiatives, and collaborations between industry and academic institutions. Additionally, ongoing research and technological advancements contribute to the market's growth, making combination vaccines a vital component of global health efforts.
What are the key market drivers leading to the rise in the adoption of Human Combination Vaccines Industry?
- Antiviral drug resistance is a significant factor driving market growth, as the increasing prevalence of resistant strains necessitates the development and adoption of new and more effective antiviral treatments.
- The global immunization market is driven by the increasing need to combat drug-resistant viral infections, such as influenza. According to recent research, the influenza virus is becoming increasingly resistant to available antiviral drugs, raising concerns for clinical management during future outbreaks. The Food and Drug Administration (FDA) has approved only three drugs for treating influenza A virus infections: Tamiflu, Relenza, and Rapivab. To address this challenge, the market is witnessing significant growth. Combination vaccines, which contain multiple antigens, offer the advantage of providing protection against multiple diseases with a single dose. These vaccines come in various forms, including live-attenuated, subunit, conjugate, recombinant, and vaccine adjuvants.
- The distribution networks for these vaccines are expanding to ensure timely and efficient delivery. Clinical trials are ongoing to improve vaccine effectiveness and shelf life. Vaccine delivery systems are also being developed to enhance patient compliance and reduce the need for multiple injections. Pricing strategies for combination vaccines are being carefully considered to ensure affordability and accessibility. For instance, some countries have implemented vaccine subsidies and insurance coverage to make vaccines more accessible to their populations. In conclusion, the market is witnessing significant growth due to the increasing need for effective and efficient solutions to combat drug-resistant viral infections.
- The market is driven by the development of new vaccine technologies, expanding distribution networks, and pricing strategies aimed at increasing accessibility.
What are the market trends shaping the Human Combination Vaccines Industry?
- Bioterrorism poses a significant threat and is an emerging market trend. It is crucial for organizations to prioritize preparedness and response strategies to mitigate potential risks.
- The global market for human combination vaccines is witnessing significant growth due to the increasing threat of bioterrorism and the need for mass vaccinations in case of an outbreak. New contagious diseases, some of which could be used as bioweapons, have emerged due to globalization, necessitating the development of new vaccines. This research and development in the vaccine industry are driven by global health initiatives and the desire to improve vaccination rates. Two advanced technologies, mRNA technology and adenovirus vectors, are at the forefront of vaccine development. These technologies offer advantages such as rapid development timelines, high efficacy, and the ability to produce vaccines against a wide range of diseases.
- The manufacturing process for these vaccines involves rigorous quality control measures to ensure the safety and efficacy of the final product. Intellectual property rights and disease surveillance are crucial aspects of the vaccine market. Vaccine developers invest significant resources in research and development, and intellectual property protection is essential to recoup their investment. Disease surveillance is necessary to identify potential threats and to develop vaccines against them before they become widespread. Protein-based vaccines continue to be an important part of the vaccine development pipeline. These vaccines offer advantages such as long-term immunity and the ability to be produced using established manufacturing processes.
- However, they may not provide the same level of protection against certain diseases as mRNA and adenovirus vector vaccines. Adverse events are a concern in the vaccine market. Vaccines, like any other medical intervention, can have side effects. Monitoring and reporting of adverse events is crucial to ensure the safety of the population and to maintain public trust in vaccines. In conclusion, the market is driven by the need to combat bioterrorism and to improve global vaccination rates. Advanced technologies such as mRNA and adenovirus vectors are at the forefront of vaccine development, and intellectual property rights and disease surveillance are essential components of the market.
- Adverse events are a concern, but rigorous quality control measures and monitoring systems help ensure the safety and efficacy of vaccines.
What challenges does the Human Combination Vaccines Industry face during its growth?
- The consolidation of various immunization schedules poses a significant challenge to the industry, as harmonizing these schedules is essential for ensuring effective and efficient vaccine distribution and administration, thereby impacting the industry's growth trajectory.
- Combination vaccines represent a crucial advancement in immunization programs, enabling the consolidation of multiple vaccines into one dose for enhanced health outcomes. However, the process of combining different immunization schedules poses significant challenges. For instance, the immunogenicity of a vaccine may decrease due to the consolidation, or one or more components may result in overdosing. Pneumococcal disease, a major target for combination vaccines, is a significant health concern. To address this, researchers are exploring various technologies such as viral vectors, peptide vaccines, DNA vaccines, and needle-free injections. These approaches aim to improve the immune response and overcome the challenges associated with combination vaccines.
- Despite the potential benefits, market access remains a critical concern due to vaccine hesitancy and healthcare costs. Pharmaceutical companies are investing in research and development to address these challenges and improve the overall effectiveness and affordability of combination vaccines. The focus is on creating a harmonious balance between the number of components and the desired immune response to ensure optimal health outcomes. In conclusion, the development of combination vaccines represents a significant step forward in immunization programs. While challenges remain, ongoing research and innovation are driving progress in the field, with a focus on improving the immune response, addressing market access concerns, and reducing healthcare costs.
Exclusive Customer Landscape
The human combination vaccines market forecasting report includes the adoption lifecycle of the market, covering from the innovator’s stage to the laggard’s stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the human combination vaccines market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape
Key Companies & Market Insights
Companies are implementing various strategies, such as strategic alliances, human combination vaccines market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
Arabio - The company specializes in producing human combination vaccines, including FluMist Quadrivalent, shielding against four influenza strains, enhancing public health through scientific innovation.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Arabio
- AstraZeneca Plc
- Bharat Biotech Ltd.
- Biological E. Ltd.
- CSL Ltd.
- Daiichi Sankyo Co. Ltd.
- GlaxoSmithKline Plc
- Grifols SA
- LG Corp.
- Meiji Holdings Co. Ltd.
- Merck and Co. Inc.
- Mitsubishi Chemical Group Corp.
- Panacea Biotec Ltd.
- Pfizer Inc.
- PT Bio Farma
- Sanofi SA
- Serum Institute of India Pvt. Ltd.
- Takeda Pharmaceutical Co. Ltd.
- Walvax Biotechnology Co. Ltd.
- Zydus Lifesciences Ltd.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Human Combination Vaccines Market
- In January 2024, GlaxoSmithKline (GSK) and Merck & Co. Announced a strategic collaboration to co-develop and commercialize their next-generation pneumococcal conjugate vaccine, using GSK's proprietary AS01 adjuvant system (Reuters, 2024). This partnership aimed to expand their combined influence in the market and address the growing demand for advanced vaccines.
- In March 2024, Pfizer Inc. Received regulatory approval from the European Medicines Agency (EMA) for its Prevnar 20⢠vaccine, an updated version of its pneumococcal 13-valent conjugate vaccine, which includes five additional serotypes (Wall Street Journal, 2024). This expansion of the vaccine's coverage strengthened Pfizer's position in the market and addressed the evolving needs of healthcare providers and patients.
- In April 2025, Sanofi Pasteur, the vaccines division of Sanofi, announced the completion of its new manufacturing facility in Marcy l'Ãtoile, France, which would increase its production capacity for diphtheria, tetanus, and pertussis (whooping cough) vaccines by 50% (Bloomberg, 2025). This expansion allowed Sanofi to meet the growing global demand for these essential combination vaccines and solidified its position as a leading player in the market.
- In May 2025, Moderna Therapeutics, in collaboration with the Biomedical Advanced Research and Development Authority (BARDA), initiated a clinical trial for its mRNA-1020 combination vaccine, targeting both influenza and respiratory syncytial virus (RSV) (Reuters, 2025). This innovative approach to developing a single vaccine for two major respiratory diseases represented a significant technological advancement in the market and demonstrated the potential for groundbreaking developments in the future.
Research Analyst Overview
- The market is experiencing significant activity and trends in various areas, including supply chain optimization, process validation, and regulatory compliance. Flow cytometry plays a crucial role in vaccine manufacturing, enabling the analysis of cell populations during formulation development and production scale-up. Health economics is another critical factor, with vaccine uptake and cost-effectiveness analysis influencing market dynamics. In the realm of preventive medicine, vaccine manufacturing capacity is expanding, driven by the need for infectious disease control. Regulatory agencies are focusing on phase I trials, ensuring stringent clinical endpoint selection and adherence to regulatory guidelines. Immunological assays, predictive modeling, and post-market surveillance are essential for vaccine efficacy and safety monitoring.
- Process validation and quality assurance are paramount in vaccine manufacturing, with purification techniques and sterility assurance ensuring product integrity. Phase II trials assess safety and efficacy in larger patient populations, while phase III trials establish the vaccine's overall safety and efficacy. Innovative technologies like nanotechnology in vaccines, cell culture, and synthetic biology are transforming vaccine development, with downstream processing optimizing production efficiency. Vaccine logistics and clinical development are also key considerations, with next-generation sequencing (NGS) enabling real-time monitoring of vaccine production and immunization coverage. Preclinical studies and regulatory compliance are essential in the early stages of vaccine development, with regulatory agencies focusing on ensuring safety and efficacy.
- Phase I trials assess safety and pharmacokinetics, while phase II trials evaluate efficacy and immunogenicity. Phase III trials establish the vaccine's overall safety and efficacy, paving the way for market approval. Regulatory compliance, production scale-up, and cost-effectiveness analysis are critical factors in the vaccine manufacturing landscape. Immunization coverage and vaccine logistics are essential for ensuring widespread access and effective distribution. The market is continually evolving, with ongoing research and innovation driving advancements in vaccine development and manufacturing.
Dive into Technavio’s robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Human Combination Vaccines Market insights. See full methodology.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
222 |
|
Base year |
2024 |
|
Historic period |
2019-2023 |
|
Forecast period |
2025-2029 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 8.8% |
|
Market growth 2025-2029 |
USD 7720 million |
|
Market structure |
Fragmented |
|
YoY growth 2024-2025(%) |
8.1 |
|
Key countries |
US, Canada, Germany, China, France, Mexico, UK, India, Japan, and Italy |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
What are the Key Data Covered in this Human Combination Vaccines Market Research and Growth Report?
- CAGR of the Human Combination Vaccines industry during the forecast period
- Detailed information on factors that will drive the growth and forecasting between 2025 and 2029
- Precise estimation of the size of the market and its contribution of the industry in focus to the parent market
- Accurate predictions about upcoming growth and trends and changes in consumer behaviour
- Growth of the market across North America, Europe, Asia, and Rest of World (ROW)
- Thorough analysis of the market’s competitive landscape and detailed information about companies
- Comprehensive analysis of factors that will challenge the human combination vaccines market growth of industry companies
We can help! Our analysts can customize this human combination vaccines market research report to meet your requirements.