Preeclampsia Laboratory Testing Market Size 2024-2028
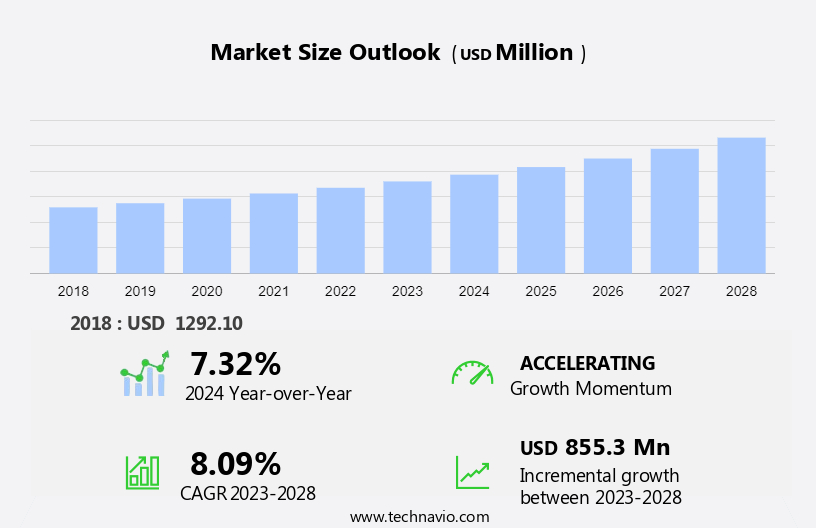
The preeclampsia laboratory testing market size is forecast to increase by USD 855.3 million at a CAGR of 8.09% between 2023 and 2028.
- The market is experiencing significant growth due to several key factors. One of the primary drivers is the expansion of the market in emerging economies, as the prevalence of preeclampsia is increasing In these regions. The market's size is significant, with a growing population and increasing demand for advanced medical technologies to address the complexities of preeclampsia diagnosis and management. Another trend influencing market growth is the growing need for companion diagnostics to improve patient outcomes and reduce healthcare costs. However, challenges persist, including limited awareness and healthcare services in developing regions, which hinder the widespread adoption of laboratory testing for preeclampsia. These factors, among others, are shaping the future of the market.
What will be the Size of the Preeclampsia Laboratory Testing Market During the Forecast Period?
- The market encompasses a range of diagnostic solutions aimed at detecting and managing this complex pregnancy complication. Preeclampsia is characterized by the onset of high blood pressure and organ dysfunction, often affecting the kidneys and liver. The market's growth is driven by several factors, including increasing teenage pregnancy rates, rising prevalence of lifestyle disorders, and the need for early detection and disease management to improve patient satisfaction and treatment adherence. Novel technologies, such as point-of-care (POC) products and personalized medicinal products, are gaining traction In the market due to their convenience and potential for improved health and safety. Infections, including malaria, can also contribute to preeclampsia, further emphasizing the importance of diagnostic capabilities. Raw materials and antenatal care also play crucial roles In the market's dynamics.
How is this Preeclampsia Laboratory Testing Industry segmented and which is the largest segment?
The preeclampsia laboratory testing industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2024-2028, as well as historical data from 2018-2022 for the following segments.
- Product Type
- Consumables
- Instruments
- Test
- Blood test
- Urine test
- Others
- Geography
- North America
- US
- Europe
- Germany
- UK
- Asia
- China
- India
- Rest of World (ROW)
- North America
By Product Type Insights
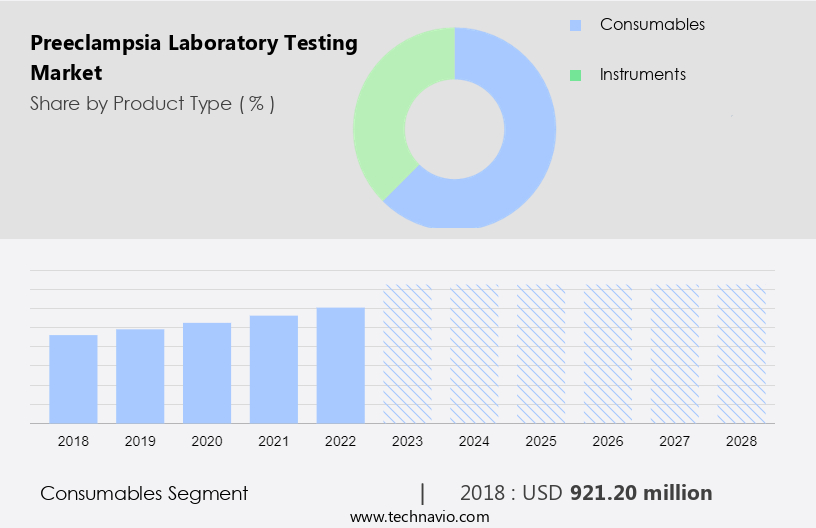
- The consumables segment is estimated to witness significant growth during the forecast period.
Preeclampsia, a pregnancy complication characterized by high blood pressure and organ dysfunction, necessitates timely and accurate diagnosis for effective disease management. Laboratories play a crucial role in preeclampsia diagnosis, utilizing various tests such as blood pressure measurement, liver and kidney function assessments, and biomarker analysis. Consumables, including reagents, are integral components of these procedures. The consumable segment's growth is driven by their frequent usage and relatively low cost.
Labs and hospitals increasingly purchase consumables online, boosting sales in this sector. Personalized medicinal products, POC (point-of-care) products, and novel technologies are emerging trends in preeclampsia diagnostics. Patient satisfaction, treatment adherence, and early detection are essential factors influencing market growth. Awareness initiatives by patient advocacy groups, health insurance policies, and antenatal care programs contribute to increased preeclampsia diagnoses. Disease management, lifestyle disorders, and medical interventions further fuel the demand for laboratory tests and diagnostic services.
Get a glance at the Preeclampsia Laboratory Testing Industry report of share of various segments Request Free Sample
The consumables segment was valued at USD 921.20 million in 2018 and showed a gradual increase during the forecast period.
Regional Analysis
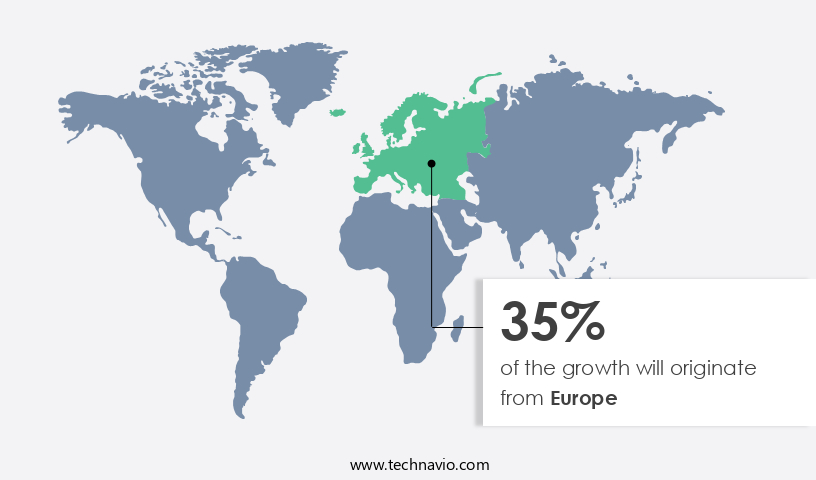
- Europe is estimated to contribute 35% to the growth of the global market during the forecast period.
Technavio's analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
For more insights on the market share of various regions, Request Free Sample
The market in North America, specifically In the US and Canada, holds a significant share due to the increasing focus on drug discovery and development, advanced biotechnology, and the presence of numerous companies offering a broad spectrum of preeclampsia testing products. The market's growth is driven by the need for accurate diagnosis and research in preeclampsia, which requires extensive laboratory testing. Factors such as rising teenage pregnancy rates, increasing awareness, and patient advocacy groups' efforts contribute to the market's expansion.
Additionally, personalized medicinal products, Point-of-Care (POC) products, and disease management are key trends In the market, prioritizing patient satisfaction, treatment adherence, and early detection. Novel technologies and lifestyle disorders also influence the market's growth, while hospitals, clinics, and diagnostic centers remain key end-users. The market's future growth is anticipated to be influenced by the increasing population, health and safety concerns, and the availability of raw materials.
Market Dynamics
Our researchers analyzed the data with 2023 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
What are the key market drivers leading to the rise In the adoption of Preeclampsia Laboratory Testing Industry?
Market expansion in emerging economies is the key driver of the market.
- The Preeclampsia Diagnostics market expansion is driven by several factors, including the rising incidence of infections during pregnancy, such as Malaria and Urinary Tract Infections (UTIs), which can contribute to preeclampsia. Additionally, the increasing rates of teenage pregnancies and the subsequent health complications increase the demand for preeclampsia laboratory testing. In emerging economies like China, India, and Brazil, this demand is further fueled by the growing prevalence of lifestyle disorders, kidney and liver dysfunction, and high-risk pregnancies. Moreover, personalized medicinal products, Point of Care (POC) products, and novel technologies are gaining popularity in disease management, enhancing patient satisfaction and treatment adherence.
- Increasing awareness of preeclampsia, driven by patient advocacy groups and health insurance policies, is also contributing to market growth. The market for preeclampsia laboratory tests is witnessing significant investments in consumables, hospitals, clinics, and diagnostic centers. The focus on early detection and precision diagnosis, using biomarkers and advanced medical technologies, is a key trend In the market. The disease pathogenesis of preeclampsia involves organ dysfunction, primarily affecting the kidneys and liver, and requires medical intervention for hospitalization and treatment. The market for preeclampsia laboratory testing is expected to continue growing due to the increasing population and the need for accurate and timely diagnosis to ensure the health and safety of pregnant women.
What are the market trends shaping the Preeclampsia Laboratory Testing Industry?
Growing need for companion diagnostics is the upcoming market trend.
- Preeclampsia, a pregnancy complication characterized by high blood pressure and organ dysfunction, primarily affects the kidneys and liver. Early detection is crucial for effective disease management and improving patient outcomes. Preeclampsia diagnostics play a significant role in this process, particularly companion diagnostics. These in-vitro point-of-care (POC) medical devices offer personalized medicinal products based on the analysis provided. The trend towards personalized medicine creates opportunities for POC products, including preeclampsia laboratory testing, to expand into new markets. These tests contribute to better prescribing decisions and improved patient satisfaction and treatment adherence. Additionally, increasing awareness of preeclampsia, driven by patient advocacy groups and health insurance policies, fuels market growth.
- Novel technologies, such as biomarkers and precision medicine, further enhance the diagnostic capabilities of these tests. Consumables, diagnostic centers, hospitals, and clinics are key players In the market. Preeclampsia diagnosis involves monitoring blood pressure, liver, and kidney function, as well as addressing potential infections, such as malaria, and lifestyle disorders. The market for preeclampsia laboratory testing is driven by the need for early detection, medical intervention, and understanding disease pathogenesis. Blood tests are a common diagnostic method, with diagnostic laboratories playing a vital role in processing and reporting results. Calcium supplements and aspirin doses are among the treatments associated with preeclampsia management.
What challenges does the Preeclampsia Laboratory Testing Industry face during its growth?
Lack of awareness and limited healthcare services in developing regions is a key challenge affecting the industry growth.
- Preeclampsia, a serious pregnancy complication characterized by high blood pressure and organ dysfunction, primarily affects pregnant women. Early detection is crucial for effective disease management and improving patient outcomes. Laboratory tests play a vital role in diagnosing preeclampsia, particularly in identifying biomarkers related to high blood pressure, liver, and kidney dysfunction. Infections, such as malaria, are linked to an increased risk of preeclampsia, especially in regions with high teenage pregnancy rates. To address this, diagnostic centers are focusing on developing novel technologies for early detection of preeclampsia and its underlying causes. Personalized medicinal products and Point of Care (POC) products are gaining popularity due to their convenience and precision. The market is driven by increasing awareness of the disease, patient advocacy groups, and health insurance policies. Patient satisfaction and treatment adherence are crucial factors influencing market growth. However, affordability and accessibility remain significant challenges, particularly in developing countries. To expand their reach, market participants must offer tests at affordable rates and collaborate with hospitals, clinics, and diagnostic centers. Raw materials suppliers and medical technology companies can also contribute by providing high-quality consumables and innovative solutions.
- The market is expected to grow as awareness of preeclampsia and its complications continues to increase, and medical interventions become more effective. Preeclampsia is a significant health and safety concern for pregnant women, with potential complications including preterm births, hospitalization, and medical intervention. By focusing on early detection, precision, and accessibility, The market can help improve disease management and patient outcomes. Blood pressure, liver, and kidney function tests are essential for diagnosing preeclampsia. Antenatal care and lifestyle disorders also play a role in disease pathogenesis. Blood tests and diagnostic laboratories are at the forefront of preeclampsia diagnosis, and novel technologies are continuously being developed to improve accuracy and accessibility. Calcium supplements and aspirin doses are common treatments for preeclampsia, but effective disease management requires a multifaceted approach. The market is poised for growth as stakeholders collaborate to improve accessibility, affordability, and accuracy of diagnostic tests.
Exclusive Customer Landscape
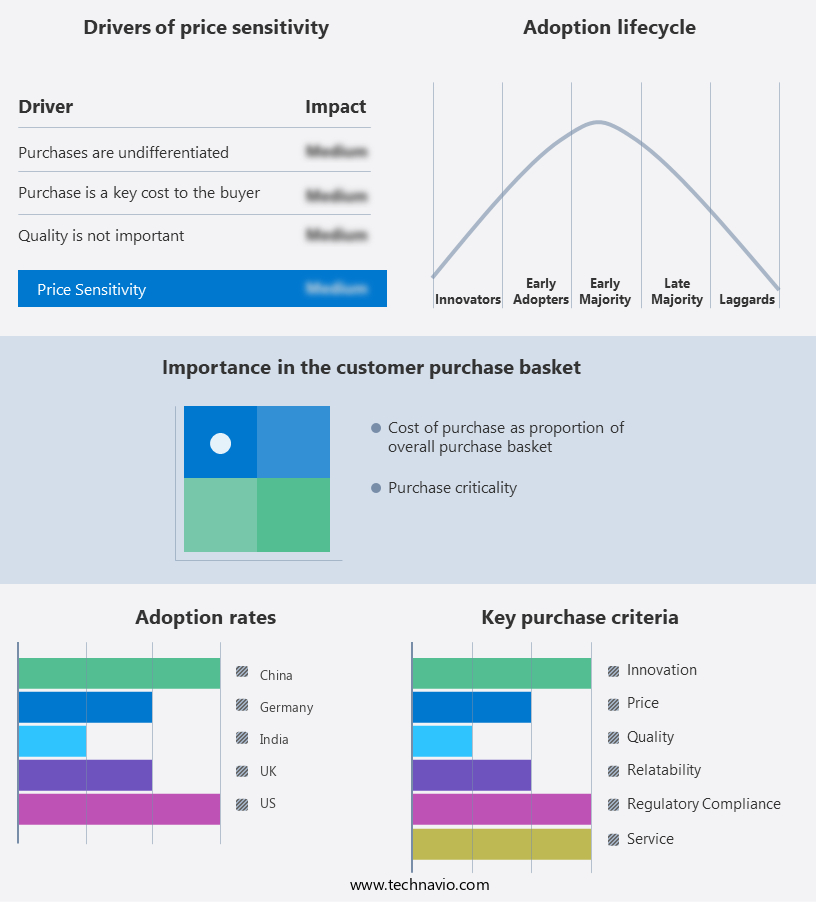
The preeclampsia laboratory testing market forecasting report includes the adoption lifecycle of the market, market growth and forecasting, covering from the innovator's stage to the laggard's stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the preeclampsia laboratory testing market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape
Key Companies & Market Insights
Companies are implementing various strategies, such as strategic alliances, preeclampsia laboratory testing market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence In the industry.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Abbott Laboratories
- ACON Laboratories Inc.
- Avecon Healthcare Pvt. Ltd.
- Bio Rad Laboratories Inc.
- BioCheck Inc.
- bioMerieux SA
- Biora Therapeutics Inc.
- Cardinal Health Inc.
- Diabetomics Inc.
- F. Hoffmann La Roche Ltd.
- GestVision Inc.
- Lifeassay Diagnostics Pty Ltd.
- MedGyn Products Inc.
- Metabolomic Diagnostics
- MOMM Diagnostics
- Perkin Elmer Inc.
- Quidelortho Corp.
- Sera Prognostics Inc.
- Siemens AG
- Thermo Fisher Scientific Inc.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Research Analyst Overview
The preeclampsia diagnostics market encompasses a range of laboratory tests designed to detect and manage this complex condition in pregnant women. Preeclampsia is a multisystem disorder characterized by new-onset high blood pressure and signs of organ dysfunction, most commonly affecting the liver and kidneys. This condition can lead to serious complications for both the mother and the developing fetus if left untreated. The market for preeclampsia diagnostics is driven by several factors. The increasing awareness of preeclampsia and its potential complications is leading to an earlier diagnosis and intervention. Novel technologies and biomarkers are being explored to improve the precision of diagnosis and enable personalized medicinal products. Infections, such as malaria and other lifestyle disorders, are known risk factors for preeclampsia. As such, diagnostic laboratories are focusing on developing point-of-care (POC) products to enable early detection and treatment in resource-limited settings. The use of POC tests can lead to improved patient satisfaction and treatment adherence, as well as reduced hospitalization and medical intervention. The disease pathogenesis of preeclampsia is not fully understood, but it is believed to involve the placental dysfunction and the release of various biomarkers into the maternal circulation.
Diagnostic centers and hospitals are investing in research and development to identify new biomarkers and improve the accuracy of existing tests. The use of raw materials In the production of consumables for preeclampsia diagnostics is a critical consideration for market players. Ensuring the quality and consistency of these materials is essential to maintaIn the accuracy and reliability of the tests. Health insurance policies play a significant role In the accessibility of preeclampsia diagnostics. Diagnostic laboratories are working to provide cost-effective solutions to make these tests more accessible to pregnant women, particularly in developing countries. Patient advocacy groups are also playing an important role in raising awareness of preeclampsia and advocating for improved access to diagnostic services. The population of pregnant women at risk for preeclampsia is significant, with an estimated 5-8% of pregnancies affected worldwide.
Early detection and intervention are crucial to prevent complications and improve outcomes for both the mother and the baby. Thus, the preeclampsia diagnostics market is a dynamic and evolving field, driven by a growing awareness of the condition and the need for early and accurate diagnosis. The use of novel technologies and biomarkers, as well as the development of cost-effective solutions, are key areas of focus for market players. The ultimate goal is to improve patient outcomes and reduce the burden of this complex condition on healthcare systems and families worldwide.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
172 |
|
Base year |
2023 |
|
Historic period |
2018-2022 |
|
Forecast period |
2024-2028 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 8.09% |
|
Market growth 2024-2028 |
USD 855.3 million |
|
Market structure |
Fragmented |
|
YoY growth 2023-2024(%) |
7.32 |
|
Key countries |
US, Germany, China, UK, and India |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
What are the Key Data Covered in this Preeclampsia Laboratory Testing Market Research and Growth Report?
- CAGR of the Preeclampsia Laboratory Testing industry during the forecast period
- Detailed information on factors that will drive the growth and forecasting between 2024 and 2028
- Precise estimation of the size of the market and its contribution of the industry in focus to the parent market
- Accurate predictions about upcoming growth and trends and changes in consumer behaviour
- Growth of the market across North America, Europe, Asia, and Rest of World (ROW)
- Thorough analysis of the market's competitive landscape and detailed information about companies
- Comprehensive analysis of factors that will challenge the preeclampsia laboratory testing market growth of industry companies
We can help! Our analysts can customize this preeclampsia laboratory testing market research report to meet your requirements.