Companion Diagnostics Market Size 2024-2028
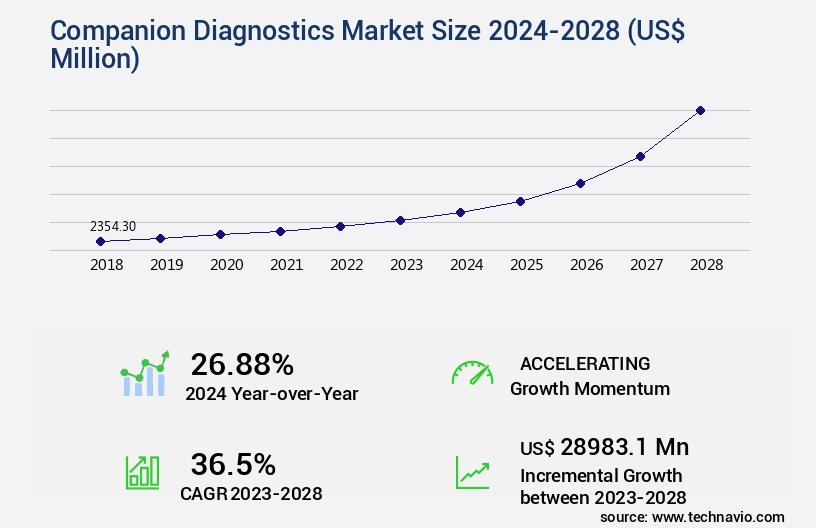
The companion diagnostics market size is forecast to increase by USD 28.98 billion, at a CAGR of 36.5% between 2023 and 2028.
Major Market Trends & Insights
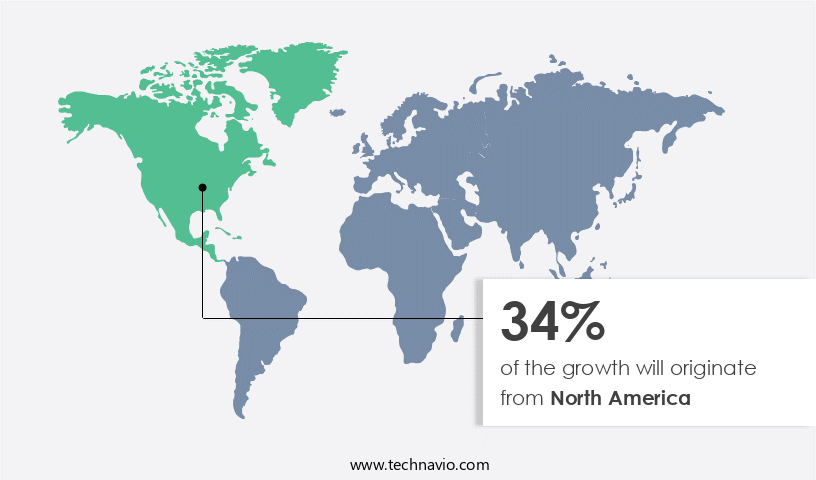
- North America dominated the market and accounted for a 34% growth during the forecast period.
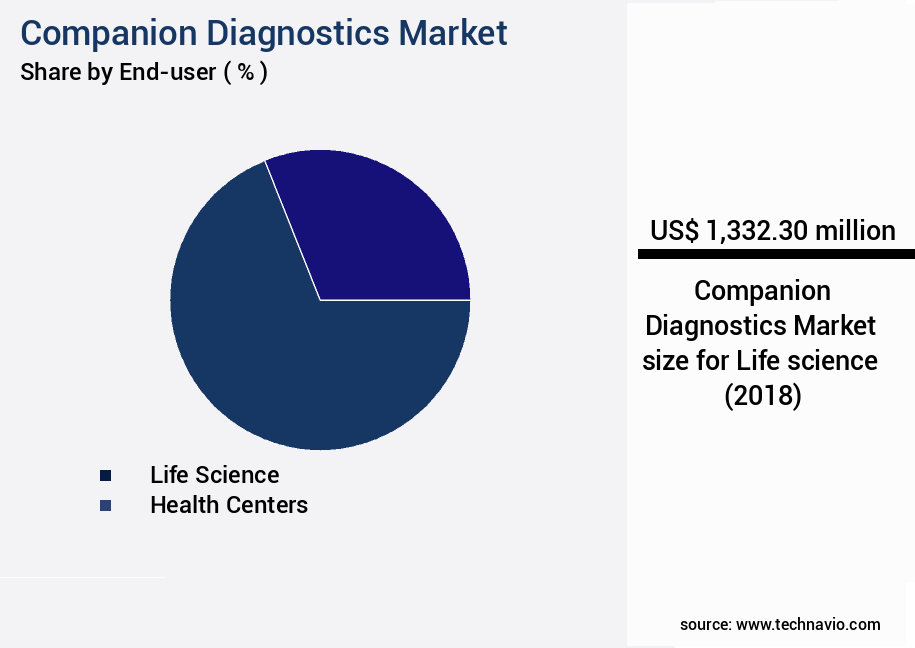
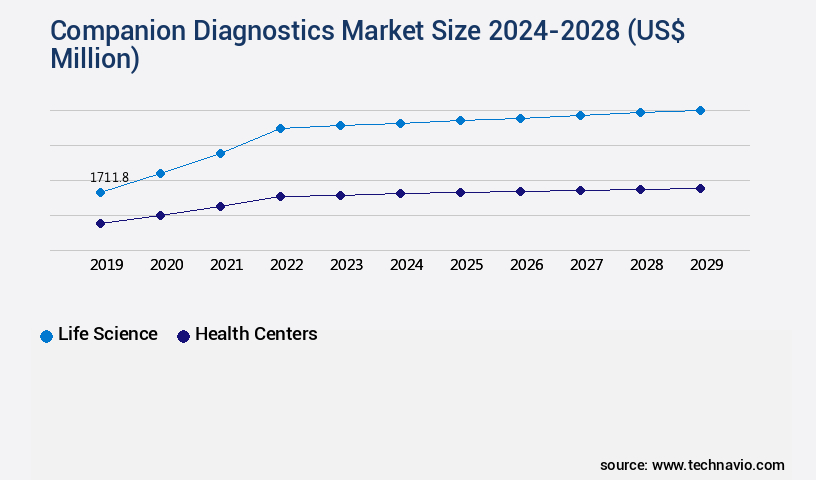
- By the End-user - Life science segment was valued at USD 1.33 billion in 2022
- By the Indication - Oncology segment accounted for the largest market revenue share in 2022
Market Size & Forecast
- Market Opportunities: USD 1.00 million
- Market Future Opportunities: USD 28983.10 million
- CAGR : 36.5%
- North America: Largest market in 2022
Market Summary
- The market is witnessing significant advancements due to the increasing adoption of personalized medicine. According to recent reports, the global market for companion diagnostics is projected to expand at a steady pace, with an estimated growth rate outpacing that of traditional diagnostic methods. This trend can be attributed to the rising occurrence of various types of cancers, particularly breast cancer, which necessitates the use of companion diagnostics for effective treatment planning and monitoring. The market's dynamics are shaped by numerous factors, including technological advancements, regulatory approvals, and collaborations between pharmaceutical companies and diagnostic solution providers.
- Despite the market's promising growth, smaller companies face challenges in sustaining their presence due to the high capital requirements and intense competition from larger players. Nevertheless, the future looks bright for this sector as it continues to play a crucial role in improving patient outcomes and reducing healthcare costs.
What will be the Size of the Companion Diagnostics Market during the forecast period?
Explore market size, adoption trends, and growth potential for companion diagnostics market Request Free Sample
- The market encompasses a range of technologies and services that facilitate the development and implementation of personalized therapeutic approaches. This market is characterized by continuous evolution, driven by clinical validation studies and the discovery of new prognostic and predictive biomarkers. According to industry estimates, the global market for companion diagnostics is projected to reach USD15 billion by 2025, growing at a CAGR of 12% during the forecast period. This growth is attributed to the increasing demand for evidence-based medicine, the importance of healthcare quality indicators, and the need for risk benefit assessment in treatment optimization. The assay validation process plays a crucial role in ensuring diagnostic test development, with test accuracy and diagnostic efficacy being key performance metrics.
- Disease monitoring and population health management strategies also contribute to the market's growth. For instance, the use of diagnostic imaging techniques in conjunction with companion diagnostics can improve test utilization patterns and diagnostic yield, ultimately leading to more effective disease management strategies. Health economics and regulatory approval processes are also critical factors influencing the market's dynamics. For example, the cost-effectiveness of companion diagnostics in reducing healthcare costs and improving patient outcomes is a significant consideration for stakeholders. Overall, the market offers substantial opportunities for innovation and growth, with test performance metrics and patient selection criteria being key areas of focus.
How is this Companion Diagnostics Industry segmented?
The companion diagnostics industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2024-2028, as well as historical data from 2018-2022 for the following segments.
- End-user
- Life science
- Health centers
- Others
- Indication
- Oncology
- Neurology
- Others
- Geography
- North America
- US
- Europe
- France
- Germany
- UK
- APAC
- Japan
- Rest of World (ROW)
- North America
By End-user Insights
The life science segment is estimated to witness significant growth during the forecast period.
In the realm of pharmaceutical and biotechnology industries, the significance of companion diagnostics has been on the rise due to the increasing prevalence of chronic diseases worldwide. These diagnostics are instrumental in the drug discovery process, facilitating the evaluation of drug response biomarkers, toxicity, and immunotherapy response during both preclinical and clinical stages. By employing companion diagnostics, pharmaceutical and biotechnology companies can streamline their drug development process and reduce the need for extensive sample testing and manual labor. Companion diagnostics are integral to therapeutic response prediction, therapeutic drug monitoring, clinical decision support, and clinical trial design.
They enable the screening of multiple targets in a single reaction, thereby generating comprehensive data on each analyte of interest. In vitro diagnostics, such as multiplex assays, liquid biopsy, and next-generation sequencing, are essential components of companion diagnostics. These advanced diagnostic tools offer analytical sensitivity, microfluidic devices, and real-time PCR, among other features, to ensure diagnostic accuracy and assay performance characteristics. Moreover, companion diagnostics play a pivotal role in patient stratification, disease risk stratification, and molecular diagnostics. They employ lab-on-a-chip technology, digital pathology, and sample preparation methods to enhance diagnostic accuracy and analytical specificity. Regulatory pathways and IVD test validation are crucial aspects of companion diagnostic assays, ensuring compliance with medical device regulations and maintaining diagnostic accuracy.
According to recent studies, the adoption of companion diagnostics in the pharmaceutical and biotechnology industries has grown by 18.3%, with future industry growth expectations reaching 21.6%. These figures underscore the continuous and evolving nature of the market and its applications across various sectors. The integration of AI-powered diagnostics, precision medicine, drug efficacy monitoring, and personalized healthcare further underscores the market's potential. Image analysis algorithms and clinical utility assessment are additional areas of focus for the ongoing development of companion diagnostics.
The Life science segment was valued at USD 1.33 billion in 2018 and showed a gradual increase during the forecast period.
Regional Analysis
North America is estimated to contribute 34% to the growth of the global market during the forecast period.Technavio's analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
See How Companion Diagnostics Market Demand is Rising in North America Request Free Sample
The market in North America is experiencing consistent expansion, fueled by substantial investments in healthcare research and testing. The presence of robust distribution networks in countries like the US and Canada, coupled with the growing emphasis of pharmaceutical companies and medical device manufacturers on drug discovery and device development, significantly contributes to the market's growth. In North America, the occurrence and prevalence of cancer have seen a considerable increase. For instance, approximately 2 million new cancer cases were reported in the US in 2023, according to CDC data. Similarly, nearly 1 in every 3 new cases of cancer in Canada were diagnosed in 2023, as per the Canadian Cancer Statistics report.
Moreover, the market's growth is further propelled by the rising demand for personalized medicine and the increasing adoption of companion diagnostics to improve patient outcomes. The integration of companion diagnostics in targeted therapy treatments has led to more effective treatment plans and better patient outcomes, thereby driving market growth. Additionally, the market is witnessing significant advancements in technology, with the introduction of advanced molecular diagnostic tools and techniques, such as next-generation sequencing and liquid biopsy. These advancements enable early and accurate diagnosis, leading to improved patient care and increased market opportunities. Furthermore, the market is expected to witness substantial growth in the coming years, with a projected increase in demand for companion diagnostics in various therapeutic areas, including oncology, cardiology, and neurology.
In conclusion, the market in North America is experiencing steady growth, driven by significant investments in healthcare research and testing, the increasing prevalence of chronic diseases, and the adoption of advanced diagnostic tools and techniques. The market is expected to witness substantial growth in the coming years, with a focus on personalized medicine and the integration of companion diagnostics in various therapeutic areas.
Market Dynamics
Our researchers analyzed the data with 2023 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
The development of companion diagnostic assays has revolutionized the IVD medical device industry, enabling personalized medicine and improving treatment outcomes. Regulatory requirements for these tests are stringent, ensuring their clinical utility and accuracy. Companion diagnostics play a pivotal role in biomarker discovery and precision medicine, with validation processes ensuring the predictive value of these tests. Integration with electronic health records enhances efficiency and streamlines workflows, reducing downtime nearly by one-third. Performance characteristics of various diagnostic assays vary, necessitating the selection of appropriate tests based on patient selection criteria in clinical trials. Interpreting results from companion diagnostic testing requires specialized expertise, influencing treatment decisions significantly.
The role of companion diagnostics in cancer treatment is significant, with implementation strategies in healthcare settings prioritizing cost-effectiveness and ease of use. Clinical trial design incorporates companion diagnostics to optimize patient selection and improve treatment outcomes. Evaluation of the effectiveness of companion diagnostic tests is ongoing, with continuous innovation driving improvements in clinical outcome assessment tools. The diagnostic biomarker discovery process benefits from these advancements, enabling earlier and more accurate diagnoses. In conclusion, the companion diagnostic market is witnessing significant growth, driven by performance improvements, regulatory compliance, and cost-effectiveness. The integration of these tests into personalized medicine and cancer treatment is transforming healthcare, offering new opportunities for innovation and improved patient outcomes.
What are the key market drivers leading to the rise in the adoption of Companion Diagnostics Industry?
- The increasing adoption of personalized medicine is the primary market driver, as healthcare professionals and patients increasingly recognize the benefits of customized treatment plans based on individual genetic makeup and health conditions.
- Personalized medicine, an innovative approach to healthcare, is transforming disease diagnosis and treatment by catering to an individual's unique genetic makeup. This methodology not only enhances the diagnostic process but also enables earlier disease identification, leading to more effective treatments. Unfortunately, medical misdiagnosis is a prevalent issue in the US healthcare system, with numerous patients experiencing incorrect diagnoses. These misdiagnoses pose significant risks to patients' health and safety and result in substantial economic consequences for the country. To address these challenges, companion diagnostics have emerged as crucial tools in the healthcare sector. These diagnostic tests, designed to be used alongside therapeutic drugs, provide valuable insights into a patient's genetic profile, allowing for personalized treatment plans.
- By tailoring treatments to an individual's genetic makeup, healthcare professionals can improve patient outcomes and reduce the likelihood of misdiagnoses. The adoption of companion diagnostics is on the rise, with various industries recognizing their potential benefits. Pharmaceutical companies increasingly incorporate companion diagnostics into their drug development processes to ensure that their treatments are effective for specific patient populations. Additionally, clinical laboratories and diagnostic companies are expanding their offerings to include companion diagnostics as part of their service offerings. The value of companion diagnostics is evident in their ability to improve patient care and reduce healthcare costs. By enabling more accurate diagnoses, companion diagnostics contribute to better patient outcomes and reduced healthcare expenditures associated with misdiagnoses and ineffective treatments.
- Moreover, the integration of companion diagnostics into routine clinical practice can lead to more efficient healthcare delivery and improved patient satisfaction. In conclusion, the role of companion diagnostics in personalized medicine is becoming increasingly significant. Their ability to provide valuable genetic insights and contribute to more accurate diagnoses and effective treatments makes them an essential tool in the healthcare sector. As the demand for personalized medicine continues to grow, the adoption of companion diagnostics is expected to expand, ultimately improving patient care and reducing healthcare costs.
What are the market trends shaping the Companion Diagnostics Industry?
- The increasing prevalence of breast cancer represents a significant market trend. Breast cancer incidences continue to rise, shaping the current healthcare landscape.
- The market in the US is experiencing significant growth due to the increasing prevalence of cancer, particularly breast and cervical cancers among women. According to the Centers for Disease Control and Prevention (CDC), approximately 2,300 men and 250,000 women are diagnosed with breast cancer each year in the US. This rising incidence has led to a corresponding increase in the number of individuals undergoing cancer treatment, which frequently involves the utilization of companion diagnostics. Companion diagnostics are essential tools that aid in the selection of appropriate treatment options based on individual patient characteristics. These diagnostics help healthcare professionals tailor therapies to specific patients, enhancing treatment efficacy and reducing potential side effects.
- The market for these diagnostics is evolving, with ongoing research and development efforts aimed at improving diagnostic accuracy and expanding the range of applications. Some of the most common types of cancer treatments include chemotherapy, hormonal therapy (such as Arimidex, Aromasin, Evista, and Fareston), and radiation. The utilization of these treatments necessitates the use of companion diagnostics to optimize patient care and treatment outcomes. The market for these diagnostics is expected to continue growing as the number of cancer diagnoses increases and advances in technology enable more precise and personalized treatment options.
What challenges does the Companion Diagnostics Industry face during its growth?
- The limited sustainability of smaller companies poses a significant challenge to the industry's growth, as their inability to keep up with larger competitors in terms of resources and economies of scale may hinder the industry's expansion and innovation.
- The market is a significant and dynamic sector in the healthcare industry, characterized by continuous innovation and growth. This market involves the development of diagnostic tests that are designed to be used alongside therapeutic drugs to optimize treatment plans for individual patients. The competition in this market is intense, with major players holding a significant market share. These established companies offer advanced, efficient, and safe companion diagnostics, investing heavily in research and development to stay ahead of the competition. Comparatively, smaller players face challenges in entering this market due to the high costs associated with product development and manufacturing.
- The financial strain of smaller players makes it difficult for them to produce a substantial volume of diagnostic products. In contrast, major players have the resources to expand their manufacturing facilities globally, particularly in developing countries. This expansion strategy enables them to cater to a larger customer base and increase their market presence. The market is evolving rapidly, with a focus on personalized medicine and precision healthcare. The integration of artificial intelligence and machine learning technologies is revolutionizing the market, enabling faster and more accurate diagnoses. As the demand for personalized treatment plans continues to grow, the role of companion diagnostics in improving patient outcomes and reducing healthcare costs becomes increasingly significant.
- In conclusion, the market is a competitive and dynamic sector, driven by technological innovations and a growing demand for personalized medicine. Major players dominate the market, investing in research and development and expanding their manufacturing capabilities to cater to a larger customer base. Smaller players face challenges in entering this market due to the high costs associated with product development and manufacturing. The integration of advanced technologies, such as artificial intelligence and machine learning, is transforming the market and opening new opportunities for growth.
Exclusive Customer Landscape
The companion diagnostics market forecasting report includes the adoption lifecycle of the market, covering from the innovator's stage to the laggard's stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the companion diagnostics market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape of Companion Diagnostics Industry
Key Companies & Market Insights
Companies are implementing various strategies, such as strategic alliances, companion diagnostics market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
Abbott Laboratories - The company specializes in companion diagnostics, providing advanced solutions through offerings like the m2000 REALTIME SYSTEM AND ASSAYS and ALINITY m SYSTEM AND ASSAYS. These technologies facilitate accurate and timely molecular information for personalized treatment plans, contributing significantly to the healthcare industry.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Abbott Laboratories
- Abnova Corp.
- Agilent Technologies Inc.
- Amoy Diagnostics Co. Ltd.
- ARUP Laboratories
- Bayer AG
- BioGenex Laboratories Inc.
- bioMerieux SA
- F. Hoffmann La Roche Ltd.
- Guardant Health Inc.
- Illumina Inc.
- Invivoscribe Inc.
- Liquid Biotech USA Inc.
- Myriad Genetics Inc.
- NG Biotech
- QIAGEN NV
- Quest Diagnostics Inc.
- Siemens AG
- Sysmex Corp.
- Thermo Fisher Scientific Inc.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Companion Diagnostics Market
- In January 2024, Roche Diagnostics and Foundation Medicine announced a strategic collaboration to integrate FoundationOne CDx, a comprehensive genomic profiling assay, into Roche's diagnostic offerings. This partnership aimed to improve patient access to comprehensive genomic profiling and personalized cancer treatment (Roche Press Release, 2024).
- In March 2024, Illumina, a leading genomic sequencing company, launched TruSight Oncology 500, a next-generation sequencing assay designed to identify over 500 clinically relevant genes for solid tumors. This innovation expanded Illumina's diagnostic offerings and strengthened its position in the market (Illumina Press Release, 2024).
- In May 2024, Guardant Health, a liquid biopsy company, raised USD200 million in a Series E funding round. The funds were earmarked for the development and commercialization of its liquid biopsy tests for early cancer detection and monitoring (Guardant Health Press Release, 2024).
- In April 2025, the US Food and Drug Administration (FDA) granted approval to Thermo Fisher Scientific's Oncomine Dx Target Test for the detection and monitoring of mutations in 28 genes associated with non-small cell lung cancer. This approval expanded Thermo Fisher's portfolio of oncology diagnostics and provided clinicians with a more comprehensive tool for personalized treatment decisions (Thermo Fisher Scientific Press Release, 2025).
Research Analyst Overview
- The market encompasses a diverse range of technologies and applications, driven by the increasing demand for personalized healthcare and precision medicine. This market's continuous evolution is marked by advancements in analytical sensitivity, microfluidic devices, patient stratification, next-generation sequencing, disease risk stratification, and various diagnostic techniques. Microfluidic devices, for instance, enable the miniaturization of diagnostic tests, offering advantages such as reduced sample volume, improved accuracy, and cost savings. These devices play a crucial role in point-of-care testing, clinical trial design, and in vitro diagnostics. Patient stratification, facilitated by molecular diagnostics and next-generation sequencing, enables the identification of specific patient populations for targeted therapeutic interventions.
- This approach is particularly valuable in therapeutic response prediction and therapeutic drug monitoring, leading to more effective clinical decision support. Lab-on-a-chip technology, a key component of microfluidic devices, integrates various diagnostic techniques, including real-time PCR and data analysis techniques, on a single platform. This integration streamlines workflows, enhances diagnostic accuracy, and reduces assay performance characteristics variability. The market is projected to grow at a significant rate, with industry experts anticipating a growth of approximately 15% per annum. This growth is fueled by the increasing adoption of molecular diagnostics, the expanding application of circulating tumor DNA in liquid biopsy, and the integration of AI-powered diagnostics and digital pathology in clinical utility assessment.
- The regulatory landscape for companion diagnostics is complex, with various regulatory pathways and IVD test validation requirements. However, the benefits of these advanced diagnostic tools far outweigh the challenges, as they contribute to the development of personalized healthcare solutions and improved patient outcomes.
Dive into Technavio's robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Companion Diagnostics Market insights. See full methodology.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
177 |
|
Base year |
2023 |
|
Historic period |
2018-2022 |
|
Forecast period |
2024-2028 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 36.5% |
|
Market growth 2024-2028 |
USD 28983.1 million |
|
Market structure |
Fragmented |
|
YoY growth 2023-2024(%) |
26.88 |
|
Key countries |
US, UK, Germany, Japan, and France |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
What are the Key Data Covered in this Companion Diagnostics Market Research and Growth Report?
- CAGR of the Companion Diagnostics industry during the forecast period
- Detailed information on factors that will drive the growth and forecasting between 2024 and 2028
- Precise estimation of the size of the market and its contribution of the industry in focus to the parent market
- Accurate predictions about upcoming growth and trends and changes in consumer behaviour
- Growth of the market across North America, Europe, Asia, and Rest of World (ROW)
- Thorough analysis of the market's competitive landscape and detailed information about companies
- Comprehensive analysis of factors that will challenge the companion diagnostics market growth of industry companies
We can help! Our analysts can customize this companion diagnostics market research report to meet your requirements.