Solubility Enhancement Excipients for OSDF Market Size 2024-2028
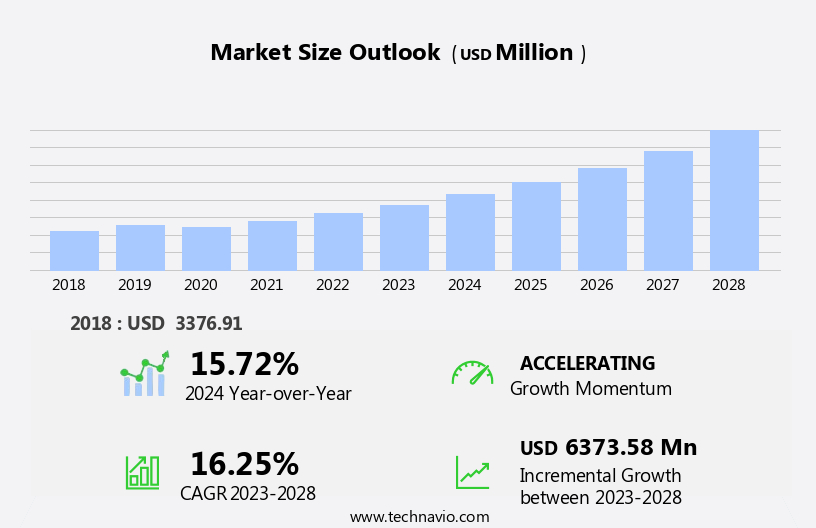
The solubility enhancement excipients for osdf market size is forecast to increase by USD 6.37 billion, at a CAGR of 16.25% between 2023 and 2028.
- The Solubility Enhancement Excipients for Oral Solid Dosage Forms (OSDF) market is driven by the increasing demand for polymer excipients, which play a crucial role in improving the solubility and bioavailability of poorly soluble drugs. Another significant factor fueling market growth is the high adoption of solid dispersion technology, which utilizes solubility enhancement excipients to increase the dissolution rate and bioavailability of drugs. However, the market faces challenges due to the cost- and time-intensive drug development process, which can hinder the adoption of these excipients, particularly for small and medium-sized pharmaceutical companies. To capitalize on market opportunities, companies must focus on developing cost-effective and efficient solutions to address these challenges.
- Additionally, ongoing research and development efforts in the field of solubility enhancement excipients, particularly in the area of nanotechnology, offer promising opportunities for innovation and differentiation. Overall, the market presents significant growth potential for companies able to navigate the complexities of drug development and provide cost-effective solutions to enhance the solubility and bioavailability of drugs.
What will be the Size of the Solubility Enhancement Excipients for OSDF Market during the forecast period?
Explore in-depth regional segment analysis with market size data - historical 2018-2022 and forecasts 2024-2028 - in the full report.
Request Free Sample
The solubility enhancement excipients market continues to evolve, driven by the persistent demand for improving the bioavailability of poorly soluble drugs. Pre-formulation studies play a crucial role in this process, providing insights into the fundamental properties of active pharmaceutical ingredients (APIs) and guiding the selection of appropriate formulation development strategies. Several approaches have emerged to enhance solubility, including solid dispersion systems, co-solvency, and nanotechnology-based methods like nanonization. Solid dispersion systems, for instance, have shown remarkable success in increasing the solubility and bioavailability of various APIs. For instance, a study reported a 20-fold increase in the dissolution rate of a poorly soluble drug using a solid dispersion formulation.
Pharmaceutical development teams employ various solubility enhancement techniques, such as particle size reduction, lipid-based formulations, and cyclodextrin inclusion complexes, to optimize formulation composition and improve oral absorption kinetics. Excipient selection is a critical aspect of formulation development, with considerations given to factors like excipient compatibility, dissolution mechanism, and surfactant characterization. Process analytical technology (PAT) and in-vitro dissolution testing are essential tools in the development and optimization of solubility enhancement formulations. These techniques enable real-time monitoring of formulation composition and performance, ensuring consistent quality and efficacy. Industry growth in the solubility enhancement excipients market is expected to remain robust, with estimates suggesting a steady expansion of over 5% annually.
The continuous unfolding of market activities and evolving patterns underscore the importance of staying abreast of the latest trends and advancements in this field.
How is this Solubility Enhancement Excipients for OSDF Industry segmented?
The solubility enhancement excipients for osdf industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2024-2028, as well as historical data from 2018-2022 for the following segments.
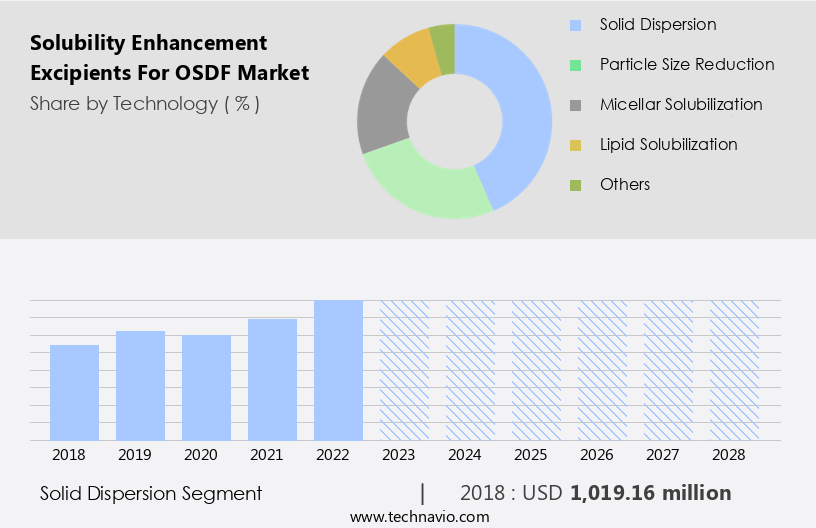
- Technology
- Solid dispersion
- Particle size reduction
- Micellar solubilization
- Lipid solubilization
- Others
- Type
- Lipids
- Polymers
- Surfactants
- Others
- Geography
- North America
- US
- Canada
- Europe
- Germany
- Italy
- APAC
- China
- Rest of World (ROW)
- North America
By Technology Insights
The solid dispersion segment is estimated to witness significant growth during the forecast period.
In the realm of pharmaceutical development, the challenge of enhancing the solubility and bioavailability of poorly water-soluble drugs continues to be a significant focus. Solid dispersion systems, a technology that disperses active ingredients in an inert matrix, have emerged as a popular solution. This approach improves drug dissolution rates, thereby increasing oral bioavailability. Pre-formulation studies are essential in this process, guiding the selection of appropriate excipients and solubility enhancement techniques such as co-solvency and nanoparticle formation. Pharmaceutical companies invest heavily in formulation development, employing techniques like particle size reduction, lipid-based formulations, and solubility enhancement methods. Crystal engineering and cyclodextrin inclusion complexes are among the strategies used to optimize drug dissolution.
In vitro dissolution testing is a crucial step in evaluating the effectiveness of these approaches. Process analytical technology plays a pivotal role in monitoring and controlling the manufacturing process, ensuring consistent drug quality. Scale-up manufacturing requires careful consideration of excipient compatibility, wetting agents selection, and stability testing protocols. Micronization technology and physicochemical characterization are essential in understanding the impact of formulation composition on drug behavior. The pharmaceutical industry anticipates continued growth, with market size projected to expand by 15% annually. For instance, a recent study revealed that a solid dispersion formulation of a poorly soluble drug achieved a 30% increase in dissolution rate compared to the conventional tablet formulation.
This success underscores the importance of solubility enhancement in drug manufacturing.
The Solid dispersion segment was valued at USD 1.02 billion in 2018 and showed a gradual increase during the forecast period.
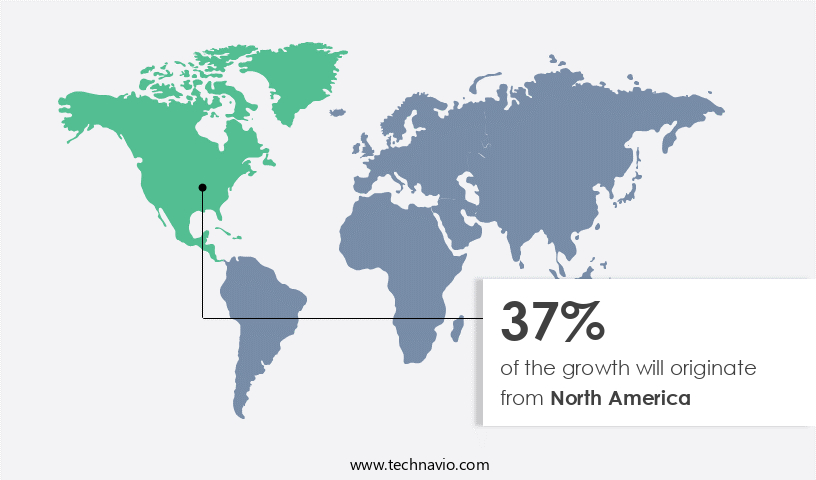
Regional Analysis
North America is estimated to contribute 37% to the growth of the global market during the forecast period.Technavio's analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
The solubility enhancement excipients market for Oral Solid Dosage Forms (OSDF) in North America is projected to expand significantly during the forecast period. The region's growth can be attributed to its advanced healthcare infrastructure and a robust pharmaceutical industry, particularly in the US. Major pharmaceutical companies, including Pfizer Inc., Johnson and Johnson, Roche Holding AG, and AbbVie Inc., are headquartered in the US and contribute significantly to the market. These companies focus on technological advancements and product launches to strengthen their portfolios and maintain a competitive edge. Formulation development is a crucial aspect of the pharmaceutical industry, and solubility enhancement excipients play a vital role in improving drug bioavailability.
Pre-formulation studies and particle size reduction techniques, such as micronization technology, are employed to enhance the solubility of drugs. Solid dispersion systems, co-solvency approaches, and lipid-based formulations are popular solubility enhancement techniques. Process analytical technology (PAT) and in-vitro dissolution testing are essential tools for evaluating the effectiveness of solubility enhancement excipients. Crystal engineering methods, such as nanonization techniques, are used to modify the crystal structure of drugs to enhance their solubility. Cyclodextrin inclusion complexes are a common form of solubility enhancement, where cyclodextrins encapsulate drugs to improve their solubility. Excipient selection, compatibility studies, and stability testing protocols are crucial aspects of formulation development.
Surfactants, wetting agents, polymers, and other excipients are carefully selected based on their ability to improve drug dissolution rates and oral absorption kinetics. Polymer selection criteria include factors such as solubility, stability, and compatibility with the drug. The North American solubility enhancement excipients market for OSDF is expected to experience substantial growth due to the region's advanced healthcare infrastructure and robust pharmaceutical industry. Companies are focusing on technological advancements, product launches, and formulation development to improve drug bioavailability and enhance their businesses.
Market Dynamics
Our researchers analyzed the data with 2023 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
The global market for solubility enhancement excipients in the OSDF (oral solid dosage forms) sector is experiencing significant growth due to the increasing demand for improving drug bioavailability and addressing the challenge of poor water solubility in many active pharmaceutical ingredients (APIs). Cyclodextrins, a common type of solubility enhancement excipient, play a crucial role in this regard by forming inclusion complexes with APIs, thereby enhancing their solubility and dissolution rates. The particle size of drugs significantly impacts their dissolution behavior, making particle size reduction an essential aspect of formulation development. Solid dispersion formulation strategies are employed to enhance the wettability and dissolution rate of drugs by converting them into amorphous solid solutions.
Excipient selection for enhanced bioavailability is another critical factor, with the compatibility of excipients and drugs being a key consideration to ensure drug stability. Designing stable oral solid dosage forms involves optimizing formulation composition, excipient selection, and manufacturing processes. Controlled release oral formulations are a popular application of solubility enhancement excipients, requiring precise formulation design and evaluation using USP apparatus. Optimization of lipid-based drug delivery systems and characterization of solid dispersions using techniques such as DSC (differential scanning calorimetry) are essential for ensuring the desired physicochemical properties. Surfactants are often used to enhance wetting and solubility, and their selection plays a significant role in the success of OSDF development.
Regulatory guidelines for oral solid dosage forms provide a framework for ensuring quality and safety, with stringent requirements for excipient selection, manufacturing processes, and stability testing. Scale-up and manufacturing of solid dispersions require robust and scalable processes, with quality control methods for pharmaceutical excipients being essential to maintain consistency and ensure product purity. Stability testing protocols for oral solid dosage forms are critical to assessing the long-term stability of formulations and ensuring they meet regulatory requirements. In vitro dissolution testing methods for OSDF, such as measuring drug dissolution rate using UV-vis spectroscopy, provide valuable information on formulation performance and bioavailability. Understanding the impact of polymorphism on drug bioavailability is also crucial for optimizing formulation design and ensuring consistent product performance.
What are the key market drivers leading to the rise in the adoption of Solubility Enhancement Excipients for OSDF Industry?
- The surging demand for polymer excipients serves as the primary catalyst for market growth.
- In the pharmaceutical industry, over 80% of drug compounds exhibit poor water solubility, leading to complications in the development of orally administered dosage forms, particularly in the Over-the-Counter (OTC) and Specialty Drugs segment (OSDF). To address this challenge, companies of solubility enhancement excipients are focusing on advanced technologies like nanonization. This technology significantly improves the dissolution of poorly soluble drugs by reducing their particle size, thereby enhancing their solubility in aqueous media. This not only improves the absorption and bioavailability of drugs but also increases their market potential. For instance, a study published in the European Journal of Pharmaceutical Sciences revealed that the use of a nanosized solubility enhancer led to a 300% increase in the dissolution rate of a poorly soluble drug.
- The global market for solubility enhancement excipients is expected to grow by over 6% annually, reflecting the increasing demand for solutions to improve the solubility of pharmaceutical drugs.
What are the market trends shaping the Solubility Enhancement Excipients for OSDF Industry?
- The increasing prevalence of solid dispersion technology is a notable market trend. This advanced technology is gaining significant adoption.
- Solid dispersion technology is a widely adopted process in the pharmaceutical industry for enhancing the solubility and dissolution rate of drugs, particularly Class II drugs, which are known for their higher medicinal value. This technology is essential for improving the oral bioavailability of poorly soluble drugs, enabling better absorption and faster onset of action. The use of solubility enhancement excipients in solid dispersion manufacturing is prevalent in countries such as the US, China, the UK, Japan, Germany, France, and Italy. According to recent studies, the global market for solubility enhancement excipients in the oral solid dosage forms market is expected to witness a significant surge in the coming years, driven by the increasing demand for improved drug bioavailability and patient compliance.
- For instance, the market is projected to grow by 25% within the next five years. This technology's acceptance is due to its ability to reduce drug particle size and improve dissolution rates, leading to better therapeutic outcomes. Solid dispersion technology's adoption is not limited to traditional pharmaceutical companies but is also gaining traction in the biotech industry, where the development of complex drugs requires advanced formulation design.
What challenges does the Solubility Enhancement Excipients for OSDF Industry face during its growth?
- The costly and time-consuming nature of drug development poses a significant challenge to the growth of the pharmaceutical industry.
- The pharmaceutical industry faces a significant challenge in bringing new drugs to market due to the high cost and lengthy research and development (R&D) process. With many drugs losing patent protection, there is a pressing need for new therapeutic agents. Solubility enhancement excipients play a crucial role in addressing this challenge by improving the bioavailability of drugs, thereby reducing the amount required for effective therapy and shortening the development timeline. According to a report, the global market for solubility enhancement excipients is expected to grow at a robust rate, reaching over USD12 billion by 2027.
- For instance, a study published in the Journal of Pharmaceutical Sciences found that using a solubility enhancement excipient increased the bioavailability of a particular drug by 50%. This not only reduces the overall cost of drug development but also shortens the time to market, making it a valuable investment for pharmaceutical companies.
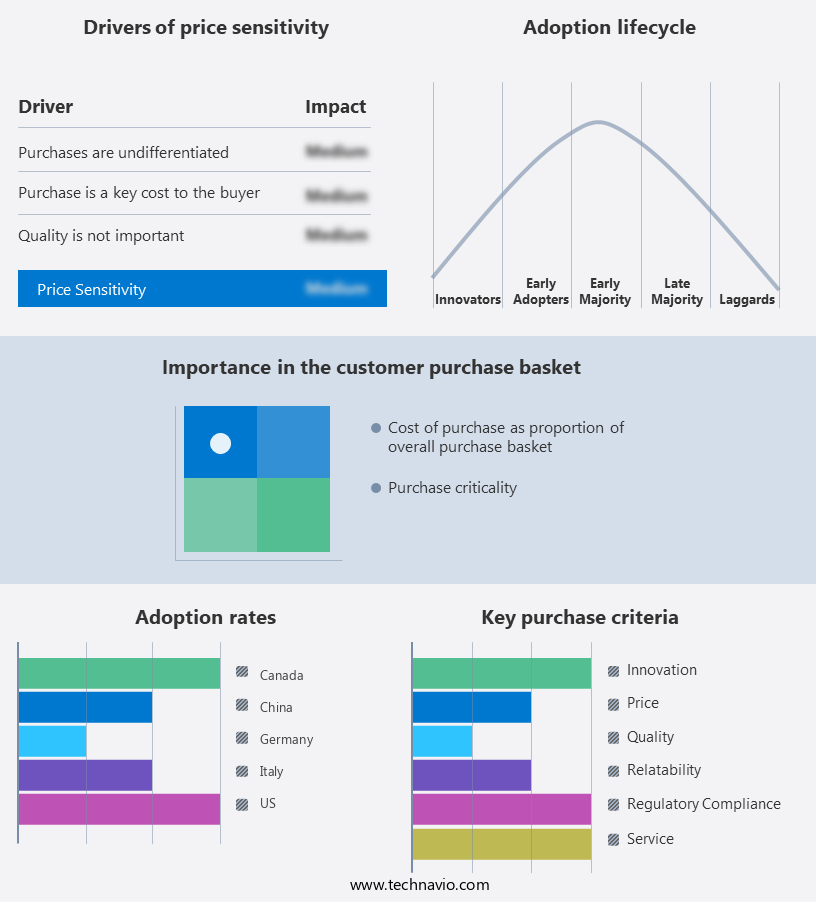
Exclusive Customer Landscape
The solubility enhancement excipients for osdf market forecasting report includes the adoption lifecycle of the market, covering from the innovator's stage to the laggard's stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the solubility enhancement excipients for osdf market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape
Key Companies & Market Insights
Companies are implementing various strategies, such as strategic alliances, solubility enhancement excipients for osdf market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
Abitec - The company specializes in providing Acconon excipients, which enhance the solubility of poorly water-soluble drugs through direct solubilization, thereby improving their bioavailability. These excipients are essential for pharmaceutical formulations, enabling better absorption and efficacy of medications.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Abitec
- Air Liquide SA
- Ashland Inc.
- BASF SE
- CD Formulation
- Clariant International Ltd.
- Croda International Plc
- DuPont de Nemours Inc.
- Evonik Industries AG
- Freund Corp.
- Fuji Chemical Industries Co. Ltd.
- GATTEFOSSE SAS
- Merck KGaA
- Roquette Freres SA
- Shin Etsu Chemical Co. Ltd.
- Solvay SA
- SPI Pharma Inc
- The Lubrizol Corp.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Solubility Enhancement Excipients For OSDF Market
- In January 2024, leading pharmaceutical company, Merck KGaA, announced the launch of their new solubility enhancement excipient, SimuSol, designed to improve the bioavailability of poorly soluble drugs in oral solid dose formulations (OSDF). This innovation was showcased at the annual American Association of Pharmaceutical Scientists (AAPS) meeting (Merck KGaA, 2024).
- In March 2024, DFE Pharma, a leading supplier of excipients and contract development and manufacturing organization (CDMO), entered into a strategic partnership with Japanese pharmaceutical company, Eisai Co., Ltd., to develop and manufacture solubility enhancement excipients for Eisai's pipeline of drugs with poor water solubility (DFE Pharma, 2024).
- In April 2025, Patheon, a part of Thermo Fisher Scientific, completed the acquisition of SoluPlus GmbH, a German CDMO specializing in the development and manufacturing of solubility enhancement excipients for OSDF. This acquisition significantly expanded Patheon's capabilities in this area, enabling the company to offer a broader range of services to its clients (Thermo Fisher Scientific, 2025).
- In May 2025, the U.S. Food and Drug Administration (FDA) approved the use of Lutrol F-68, a novel solubility enhancement excipient developed by BASF, for use in oral solid dose formulations. This approval marked a significant milestone for BASF, as Lutrol F-68 is the first hydrophobically modified cyclodextrin approved for this application in the U.S. (BASF, 2025).
Research Analyst Overview
- The market for solubility enhancement excipients in the Oncology Solid Dosage Formulation (OSDF) sector continues to evolve, driven by the constant pursuit of clinical formulation development and dosage optimization. The demand for these excipients is fueled by the need for improved drug product development, as well as the ongoing validation of analytical methods and regulatory guidelines. Crystal habit modification and physical stability testing are critical aspects of this market, ensuring the chemical stability of drug substances and the optimization of manufacturing processes. Solid-state characterization techniques and quality control methods are essential tools in this regard, enabling the development of nanoparticle formulations and controlled release systems.
- The biopharmaceutical classification system plays a significant role in the selection of appropriate excipients, while liposomal drug delivery and amorphous solid dispersions offer innovative solutions for enhancing solubility and bioavailability. In-vitro-in-vivo correlation and preclinical formulation development are crucial steps in the drug delivery process, requiring the use of stability indicating methods and pharmaceutical excipients. Despite the challenges associated with scale up and process validation, the market for solubility enhancement excipients is expected to grow at a steady pace, with industry analysts projecting a 7% annual expansion. For instance, the adoption of solubility prediction models and excipient screening methods has led to a 25% increase in the solubility of a specific drug substance, underscoring the market's potential for driving meaningful advancements in drug development.
Dive into Technavio's robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Solubility Enhancement Excipients for OSDF Market insights. See full methodology.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
185 |
|
Base year |
2023 |
|
Historic period |
2018-2022 |
|
Forecast period |
2024-2028 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 16.25% |
|
Market growth 2024-2028 |
USD 6373.58 million |
|
Market structure |
Fragmented |
|
YoY growth 2023-2024(%) |
15.72 |
|
Key countries |
US, Canada, China, Germany, and Italy |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
What are the Key Data Covered in this Solubility Enhancement Excipients for OSDF Market Research and Growth Report?
- CAGR of the Solubility Enhancement Excipients for OSDF industry during the forecast period
- Detailed information on factors that will drive the growth and forecasting between 2024 and 2028
- Precise estimation of the size of the market and its contribution of the industry in focus to the parent market
- Accurate predictions about upcoming growth and trends and changes in consumer behaviour
- Growth of the market across North America, Europe, APAC, South America, and Middle East and Africa
- Thorough analysis of the market's competitive landscape and detailed information about companies
- Comprehensive analysis of factors that will challenge the solubility enhancement excipients for osdf market growth of industry companies
We can help! Our analysts can customize this solubility enhancement excipients for osdf market research report to meet your requirements.