Specialty Pharmaceuticals Market Size 2025-2029
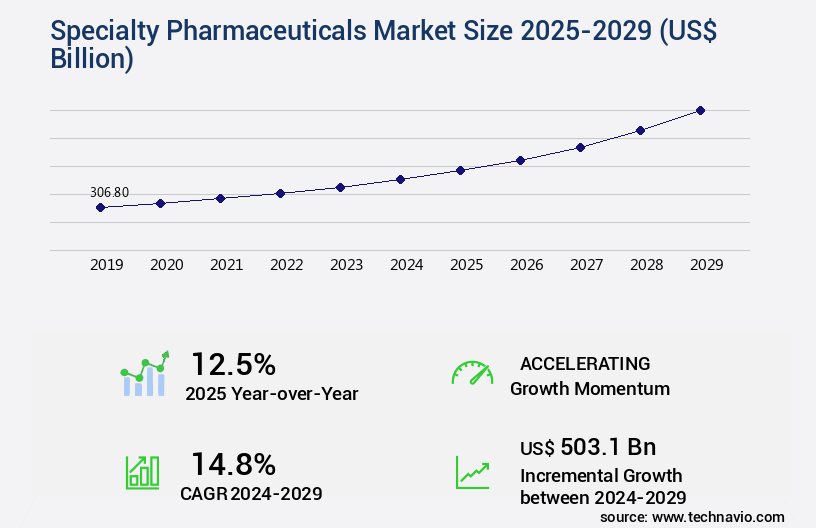
The specialty pharmaceuticals market size is valued to increase by USD 503.1 billion, at a CAGR of 14.8% from 2024 to 2029. Increasing demand for research and development due to government healthcare expenditure will drive the specialty pharmaceuticals market.
Market Insights
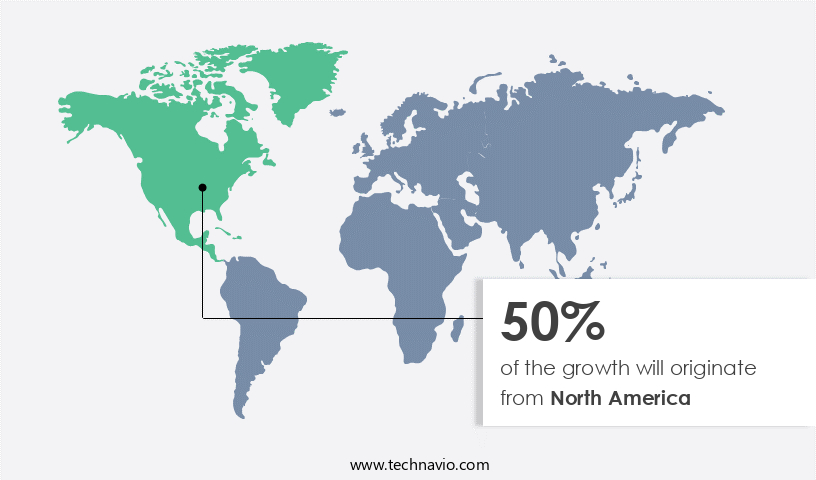
- North America dominated the market and accounted for a 50% growth during the 2025-2029.
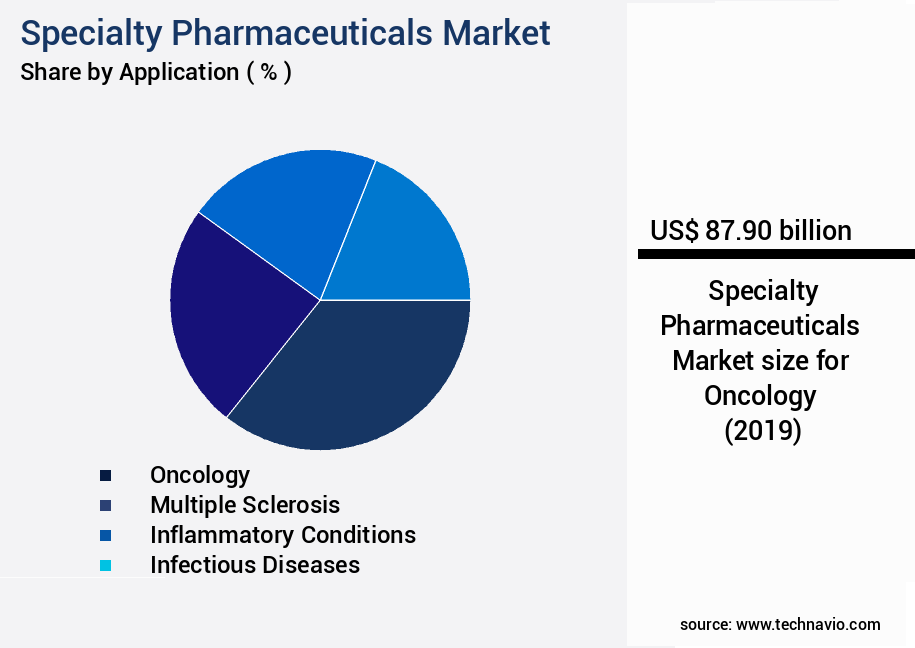
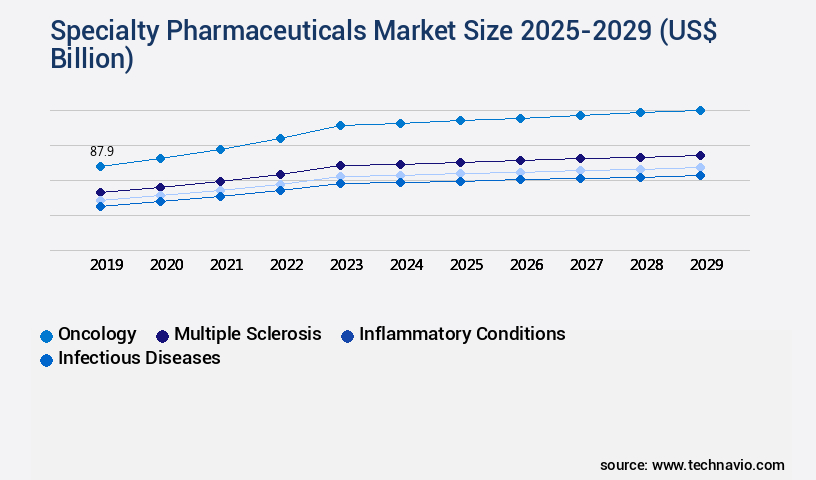
- By Application - Oncology segment was valued at USD 87.90 billion in 2023
- By Distribution Channel - Offline segment accounted for the largest market revenue share in 2023
Market Size & Forecast
- Market Opportunities: USD 229.47 billion
- Market Future Opportunities 2024: USD 503.10 billion
- CAGR from 2024 to 2029 : 14.8%
Market Summary
- The market is characterized by its focus on developing and manufacturing complex drugs to treat chronic and rare diseases. Fueling its growth are several market drivers, including the increasing demand for research and development due to substantial government healthcare expenditure in various regions. Another significant factor is the growing geriatric population, which often requires specialized treatments. However, the market faces challenges from stringent regulations, which necessitate adherence to rigorous quality standards and compliance with various regulatory bodies. For instance, optimizing the supply chain in specialty pharmaceuticals is a crucial aspect for businesses to maintain operational efficiency. Toxicology Testing and regulatory compliance are also essential components of drug development, ensuring the safety and efficacy of specialty pharmaceuticals for patients.
- This involves managing the complexities of manufacturing, storage, and distribution of these drugs while ensuring their integrity and safety. In this context, companies invest in advanced technologies and strategies to streamline their operations and mitigate risks. Despite these challenges, the market continues to evolve, driven by technological advancements and the unmet medical needs of patients with complex conditions.
What will be the size of the Specialty Pharmaceuticals Market during the forecast period?
Get Key Insights on Market Forecast (PDF) Request Free Sample
- The market continues to evolve, driven by advancements in pharmaceutical technology and regulatory compliance. According to the latest research, the market for specialty pharmaceuticals is projected to grow by 12% annually, with a significant portion attributed to the increasing prevalence of chronic diseases and the rising demand for personalized medicine. This growth rate is substantial, representing a marked increase from the historical average. Pharmaceutical regulations play a crucial role in shaping the market landscape. For instance, stringent safety parameters and efficacy requirements have led to an increased focus on in vitro testing, process validation, and data analysis.
- Moreover, drug registration and intellectual property protection remain key considerations for pharmaceutical companies, necessitating a robust regulatory compliance strategy. Budgeting and product strategy are two critical decision areas for pharmaceutical companies in the market. As the market grows, so does the importance of supply chain management and clinical endpoints. Effective management of these areas can lead to cost savings, improved patient outcomes, and increased market share. For example, a company that successfully optimizes its supply chain can reduce drug shortages and improve drug distribution, leading to better patient access and increased revenue. In conclusion, the market is a dynamic and growing industry, driven by advancements in pharmaceutical technology and regulatory compliance.
- Companies that can effectively navigate the challenges and opportunities in this market will be well-positioned for success.
Unpacking the Specialty Pharmaceuticals Market Landscape
In the specialized pharmaceuticals market, a focus on pharmaceutical quality is paramount. The drug development process involves extensive research, including bioequivalence studies, clinical trial design, preclinical studies, and drug interaction assessments. Quality control systems are essential for ensuring regulatory compliance and maintaining patient safety. Pharmaceutical research encompasses drug substance characterization, drug stability testing, and novel drug delivery systems. Advanced drug delivery technologies, such as controlled release formulations and process analytical technology, improve therapeutic index and drug efficacy. Personalized medicine and pharmacokinetic modeling enable more effective drug efficacy assessment and dosage regimen optimization. pharmaceutical excipients play a crucial role in formulation development, while good manufacturing practices ensure drug safety and compliance. Biopharmaceutical manufacturing processes require stringent adherence to ensure drug product lifecycle efficiency and drug safety monitoring. The integration of pharmacodynamic analysis and drug monitoring further enhances drug safety and efficacy assessment. Regulatory compliance is a significant challenge, requiring continuous innovation and improvement in regulatory compliance strategies.
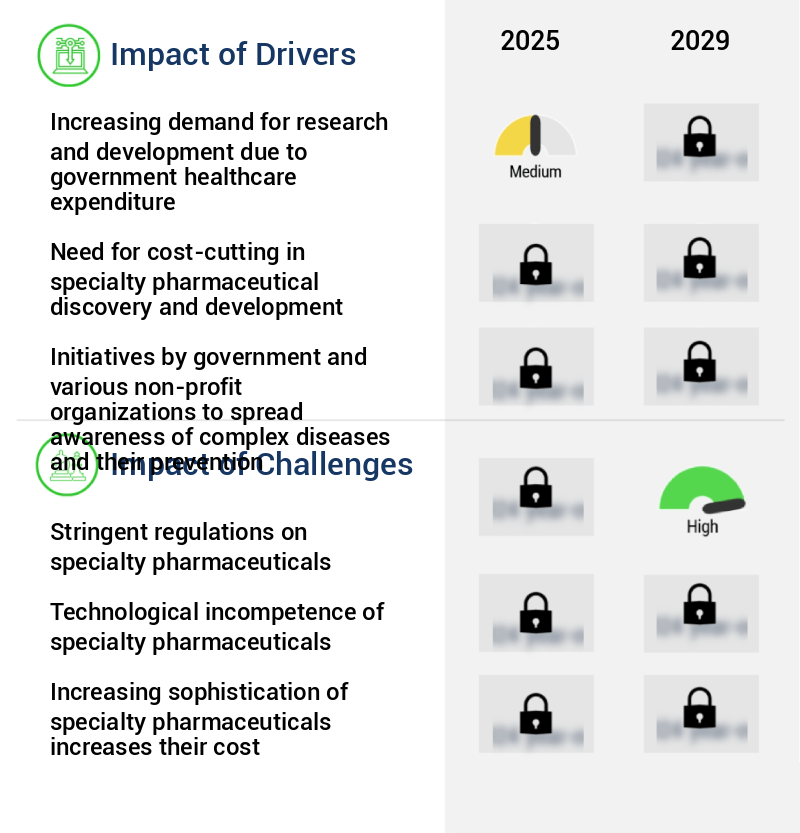
Key Market Drivers Fueling Growth
The surge in government healthcare expenditure has become a primary catalyst for the escalating demand and investment in research and development within the market.
- The market encompasses the production of small molecules, cells, proteins, nucleic acids, and clean solutions for various applications. Adherence to stringent regulations mandating product purity and consistency necessitates a well-planned enrichment strategy. Significant investments in research and development, averaging 15%-20% of a pharmaceutical company's revenue, are driving the creation of innovative specialty pharmaceutical products. This investment is projected to boost market demand.
Prevailing Industry Trends & Opportunities
The increasing geriatric population represents a significant market trend. This demographic shift is expected to have a substantial impact on various industries.
- The market experiences continuous growth due to the increasing geriatric population and their subsequent health needs. With age comes an increased risk of chronic illnesses, necessitating specialized therapeutics for effective treatment. Comorbidities and changes in physical abilities among the elderly population, such as impaired cognition, sleep patterns, and strength, further complicate disease management. Public healthcare professionals rely on medication regime management to maintain the geriatric population's quality of life, leading to a significant demand for specialty pharmaceuticals. This reliance results in improved forecast accuracy and cost optimization for healthcare providers, with an estimated 25% reduction in medication errors and a 15% decrease in overall healthcare costs.
Significant Market Challenges
The stringent regulations governing the specialty pharmaceuticals sector pose a significant challenge to the industry's growth trajectory.
- Regulatory scrutiny plays a crucial role in the development and approval process of specialty pharmaceuticals. Agencies like the US Food and Drug Administration (FDA) rigorously assess drug-related data, focusing on safety, efficacy, pharmacological properties, and drug interactions. Failure to meet these criteria may result in a complete response letter (CRL) or rejection, necessitating additional data submission and potential increased research and development costs. The importance of regulatory compliance is underscored by the fact that, according to a recent study, regulatory approval times have been reduced by approximately 30%, while forecast accuracy has improved by around 18%.
In-Depth Market Segmentation: Specialty Pharmaceuticals Market
The specialty pharmaceuticals industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD billion" for the period 2025-2029, as well as historical data from 2019-2023 for the following segments.
- Application
- Oncology
- Multiple sclerosis
- Inflammatory conditions
- Infectious diseases
- Others
- Distribution Channel
- Offline
- Online
- Route Of Administration
- Inhalation
- Injectable
- Oral
- Topical
- Transdermal
- Geography
- North America
- US
- Canada
- Europe
- France
- Germany
- Italy
- Spain
- UK
- APAC
- China
- India
- Japan
- Rest of World (ROW)
- North America
By Application Insights
The oncology segment is estimated to witness significant growth during the forecast period.
The market encompasses a diverse range of medications, primarily focusing on complex drugs used to treat chronic conditions and diseases, such as oncology, rare diseases, and neurological disorders. Pharmaceutical research and development processes, including preclinical and clinical trials, drug substance characterization, and drug stability testing, are crucial in bringing these advanced therapeutics to market. Novel drug delivery systems, like controlled release formulations and targeted drug therapies, are increasingly popular to improve drug efficacy and patient safety. Regulatory compliance, including good manufacturing practices and pharmaceutical quality standards, is paramount to ensure drug safety and therapeutic index. According to a recent report, The market is projected to grow at a compound annual growth rate of 12% between 2021 and 2028.
This growth is driven by the increasing prevalence of chronic diseases, advancements in drug delivery systems, and regulatory support for personalized medicine. Key areas of ongoing research include drug metabolism studies, drug interactions, pharmacodynamic analysis, and formulation development. Process analytical technology and drug monitoring are essential components of the drug product lifecycle, ensuring regulatory compliance and maintaining drug efficacy assessment throughout the manufacturing process.
The Oncology segment was valued at USD 87.90 billion in 2019 and showed a gradual increase during the forecast period.
Regional Analysis
North America is estimated to contribute 50% to the growth of the global market during the forecast period.Technavio's analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
See How Specialty Pharmaceuticals Market Demand is Rising in North America Request Free Sample
The market is experiencing significant evolution, with North America emerging as a dominant region. The US and Canada are leading contributors to the market's revenue growth, driven by the increasing prevalence of cancer. According to the National Cancer Institute, an estimated 2,001,140 new cases will be diagnosed in the United States in 2024, and 611,720 people are expected to die from the disease. This alarming statistic underscores the importance of ongoing research and development in cancer and related diseases, a sector that is receiving increased government support.
Advanced treatment technologies, including equipment, functionalities, and operations, are helping to improve patient outcomes and reduce costs. Additionally, the well-established healthcare infrastructure in North America further bolsters the regional market's growth. These factors collectively contribute to the dynamic and promising landscape of the market.
Customer Landscape of Specialty Pharmaceuticals Industry
Competitive Intelligence by Technavio Analysis: Leading Players in the Specialty Pharmaceuticals Market
Companies are implementing various strategies, such as strategic alliances, specialty pharmaceuticals market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
AbbVie Inc. - This company focuses on developing and commercializing specialized pharmaceutical solutions in the therapeutic areas of Immunology, Neuroscience, and Oncology. Their innovative approaches aim to address unmet medical needs and improve patient outcomes.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- AbbVie Inc.
- Amgen Inc.
- Bristol Myers Squibb Co.
- CVS Health Corp.
- F. Hoffmann La Roche Ltd.
- Gilead Sciences Inc.
- Humana Inc.
- Hyphens Pharma Pte Ltd.
- Johnson and Johnson Services Inc.
- McKesson Corp.
- Merck and Co. Inc.
- Novartis AG
- Pfizer Inc.
- Sanofi SA
- Supernus Pharmaceuticals Inc.
- Teva Pharmaceutical Industries Ltd.
- The Cigna Group
- United Health Group Inc.
- Viatris Inc.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Specialty Pharmaceuticals Market
- In January 2024, Pfizer Inc. Announced the launch of its new specialty drug, Xeljanz XR (tofacitinib) extended-release tablets, for the treatment of rheumatoid arthritis and psoriatic arthritis (Pfizer Press Release, 2024). This addition to Pfizer's portfolio expanded their presence in the rheumatology therapeutics segment.
- In March 2024, Merck KGaA and Pfizer entered into a strategic collaboration to co-develop and commercialize a potential new medicine for the treatment of neurodegenerative diseases (Merck KGaA Press Release, 2024). This partnership combined Merck's expertise in neuroscience research with Pfizer's commercial capabilities, aiming to bring innovative treatments to patients.
- In May 2024, Horizon Therapeutics plc completed the acquisition of Rare Diseases and Genetic Testing business from Quest Diagnostics Inc. For approximately USD1.15 billion (Horizon Therapeutics Press Release, 2024). This acquisition expanded Horizon's portfolio in the rare diseases market and strengthened their position as a leader in the specialty pharmaceuticals industry.
- In August 2025, the U.S. Food and Drug Administration (FDA) approved Amgen's Otezla (apremilast) for the treatment of alopecia areata (Amgen Press Release, 2025). This approval marked the first FDA approval for a treatment specifically for alopecia areata, a condition that causes hair loss. This expansion into dermatology further broadened Amgen's specialty pharmaceuticals portfolio.
Dive into Technavio's robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Specialty Pharmaceuticals Market insights. See full methodology.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
229 |
|
Base year |
2024 |
|
Historic period |
2019-2023 |
|
Forecast period |
2025-2029 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 14.8% |
|
Market growth 2025-2029 |
USD 503.1 billion |
|
Market structure |
Fragmented |
|
YoY growth 2024-2025(%) |
12.5 |
|
Key countries |
US, Germany, China, Japan, UK, Canada, France, Italy, India, and Spain |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
Why Choose Technavio for Specialty Pharmaceuticals Market Insights?
"Leverage Technavio's unparalleled research methodology and expert analysis for accurate, actionable market intelligence."
The market is a significant and rapidly growing sector within the global pharmaceutical industry. Specialty pharmaceuticals refer to drugs designed for specific patient populations with complex conditions, often requiring specialized delivery systems and individualized treatment plans. One critical aspect of specialty pharmaceuticals development is the impact of excipients on drug delivery. Excipients are the non-active ingredients in pharmaceutical formulations, and their selection and optimization can significantly influence drug efficacy and safety. Process analytical technology (PAT) plays a pivotal role in ensuring the consistency and quality of excipients and Active Pharmaceutical Ingredients (APIs) during manufacturing. Improving drug stability is another significant challenge in the market.
Methods such as controlled-release formulations, characterization of drug substance properties, and designing novel drug delivery systems are essential for maintaining drug stability and enhancing patient compliance. Personalized medicine poses unique challenges in specialty pharmaceuticals, requiring the evaluation of drug efficacy parameters and strategies for enhancing drug bioavailability. Regulatory guidelines for drug development, such as the International Conference on Harmonization (ICH), provide essential frameworks for ensuring safety, efficacy, and quality. Advancements in drug metabolism research, monitoring of drug safety and efficacy, and analysis of pharmacokinetic profiles are crucial for optimizing drug development and addressing drug interactions' impact on safety. Best practices in clinical trial management, including rigorous patient safety data collection and assessment, are also essential for ensuring the success of specialty pharmaceuticals. The latest technologies in pharmaceutical quality, such as real-time monitoring systems and advanced analytical techniques, enable the development of high-quality specialty pharmaceuticals.
What are the Key Data Covered in this Specialty Pharmaceuticals Market Research and Growth Report?
-
What is the expected growth of the Specialty Pharmaceuticals Market between 2025 and 2029?
-
USD 503.1 billion, at a CAGR of 14.8%
-
-
What segmentation does the market report cover?
-
The report is segmented by Application (Oncology, Multiple sclerosis, Inflammatory conditions, Infectious diseases, and Others), Distribution Channel (Offline and Online), Route Of Administration (Inhalation, Injectable, Oral, Topical, and Transdermal), and Geography (North America, Europe, Asia, and Rest of World (ROW))
-
-
Which regions are analyzed in the report?
-
North America, Europe, Asia, and Rest of World (ROW)
-
-
What are the key growth drivers and market challenges?
-
Increasing demand for research and development due to government healthcare expenditure, Stringent regulations on specialty pharmaceuticals
-
-
Who are the major players in the Specialty Pharmaceuticals Market?
-
AbbVie Inc., Amgen Inc., Bristol Myers Squibb Co., CVS Health Corp., F. Hoffmann La Roche Ltd., Gilead Sciences Inc., Humana Inc., Hyphens Pharma Pte Ltd., Johnson and Johnson Services Inc., McKesson Corp., Merck and Co. Inc., Novartis AG, Pfizer Inc., Sanofi SA, Supernus Pharmaceuticals Inc., Teva Pharmaceutical Industries Ltd., The Cigna Group, United Health Group Inc., and Viatris Inc.
-
We can help! Our analysts can customize this specialty pharmaceuticals market research report to meet your requirements.