US Controlled Substance Market Size 2026-2030
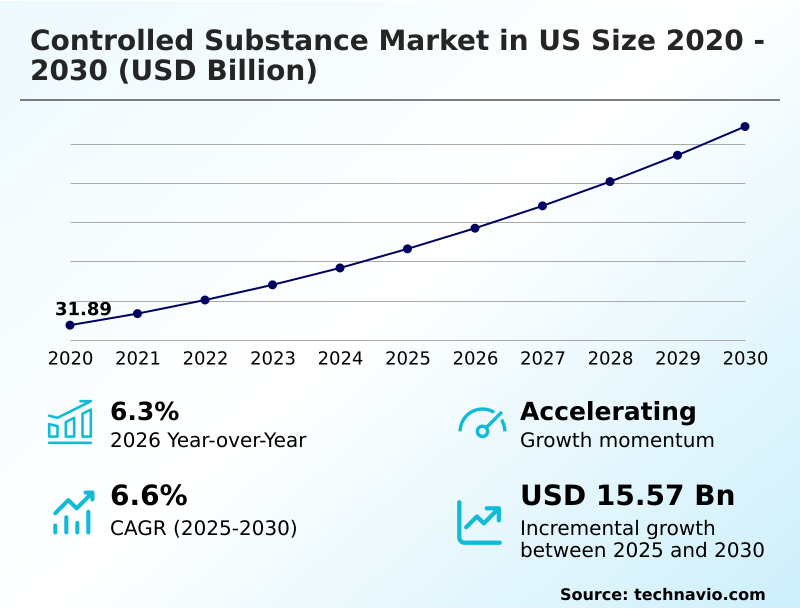
The us controlled substance market size is valued to increase by USD 15.57 billion, at a CAGR of 6.6% from 2025 to 2030. High prevalence of chronic diseases and an aging population will drive the us controlled substance market.
Major Market Trends & Insights
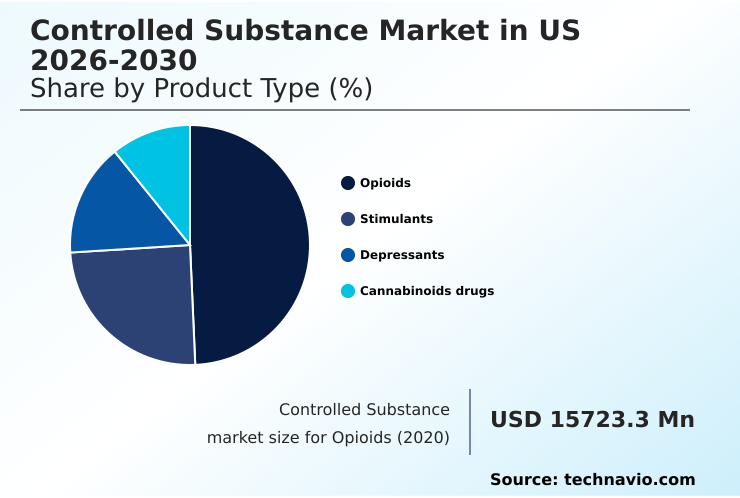
- By Product Type - Opioids segment was valued at USD 19.47 billion in 2024
- By Indication - Pain management segment accounted for the largest market revenue share in 2024
Market Size & Forecast
- Market Opportunities: USD 25.27 billion
- Market Future Opportunities: USD 15.57 billion
- CAGR from 2025 to 2030 : 6.6%
Market Summary
- The controlled substance market in US is defined by a critical balance between providing essential therapies and mitigating significant public health risks. It encompasses a range of medications, from opioid analgesics for severe pain to CNS stimulants for neurological disorders, all governed by the controlled substances act (CSA).
- A primary driver is the persistent clinical need within an aging population for effective pain management pathways. However, the market operates under intense regulatory pressure, with DEA quotas strictly limiting production volumes of schedule II drugs and other psychotropic substances. A key trend is the industry's focus on innovation in abuse-deterrent formulations and controlled drug delivery systems to enhance safety.
- For instance, a contract development and manufacturing organization (CDMO) specializing in GMP manufacturing of sedative-hypnotics must navigate complex supply chain security protocols and clinical trial regulations to ensure cGMP compliance and prevent diversion.
- This operational reality is compounded by the ongoing development of non-opioid alternatives and digital therapeutics, which are reshaping long-term treatment paradigms for conditions like chronic pain and substance use disorder (SUD) treatment. The market's trajectory depends on balancing medical access with robust drug diversion prevention and pharmacovigilance.
What will be the Size of the US Controlled Substance Market during the forecast period?
Get Key Insights on Market Forecast (PDF) Request Free Sample
How is the US Controlled Substance Market Segmented?
The us controlled substance industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2026-2030, as well as historical data from 2020-2024 for the following segments.
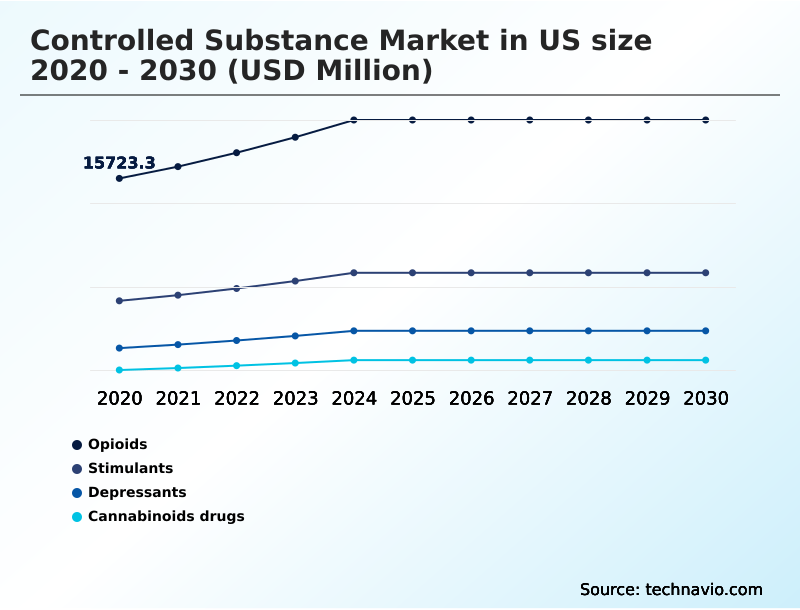
- Product type
- Opioids
- Stimulants
- Depressants
- Cannabinoids drugs
- Indication
- Pain management
- Sleep disorder
- Seizure
- Others
- Distribution channel
- Hospital pharmacies
- Retail pharmacies
- Online pharmacies
- Geography
- North America
- US
- North America
By Product Type Insights
The opioids segment is estimated to witness significant growth during the forecast period.
The opioids segment remains a significant and highly scrutinized area, centered on potent opioid analgesics for managing moderate to severe pain. These substances are a first-line therapy in settings like oncology and palliative medicine.
The market is shaped by the public health crisis, leading to stringent oversight and a push for abuse-deterrent formulations. This includes physical and chemical barriers to prevent misuse.
Regulatory bodies set aggregate production quotas (APQ) to control supply, while new pain management pathways and an emphasis on multimodal analgesia are altering prescribing habits.
These dynamics compel manufacturers to focus on risk evaluation and mitigation strategies (REMS), impacting the development of new active pharmaceutical ingredients (APIs) and requiring advanced therapeutic equivalence studies for generics.
The shift has increased the focus on patient assistance programs to ensure access for legitimate needs.
The Opioids segment was valued at USD 19.47 billion in 2024 and showed a gradual increase during the forecast period.
Market Dynamics
Our researchers analyzed the data with 2025 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
- Successfully navigating the controlled substance market in us 2026-2030 hinges on a granular understanding of its intricate dynamics. A central operational issue is the impact of DEA quotas on ADHD medication, which creates significant supply chain challenges for generic stimulants and affects patient access.
- Concurrently, the role of PDMPs in curbing opioid misuse continues to expand, forcing companies to refine compliance strategies. Significant R&D investment is directed toward abuse-deterrent technology for opioid analgesics, a key differentiator in a crowded market. The industry is also closely watching evolving telemedicine regulations for schedule II drugs, which could redefine care delivery models.
- For manufacturers, understanding the clinical efficacy of cannabinoid-based epilepsy drugs and the complex regulatory pathways for digital therapeutics is crucial for future growth. Addressing challenges in controlled substance logistics requires robust systems to manage secure transport and storage. Innovations in non-addictive pain management and emerging pain management pathways represent both a competitive threat and an opportunity for diversification.
- Firms leveraging advanced analytics to monitor supply chain vulnerabilities report a 20% faster response time to potential shortages. This proactive stance is essential for navigating the complexities of synthetic cannabinoid API manufacturing standards, pharmacovigilance for novel psychotropic substances, and the development of buprenorphine formulations for addiction therapy.
- Long-term strategic planning must also account for advances in postpartum depression pharmacotherapy, the long-term effects of sedative-hypnotics, and the development of neuroactive steroid drugs and GABA receptor modulators for anxiety disorders. Finally, REMS program compliance for specialty generics and telehealth impact on mental health parity remain critical considerations in this tightly regulated field.
What are the key market drivers leading to the rise in the adoption of US Controlled Substance Industry?
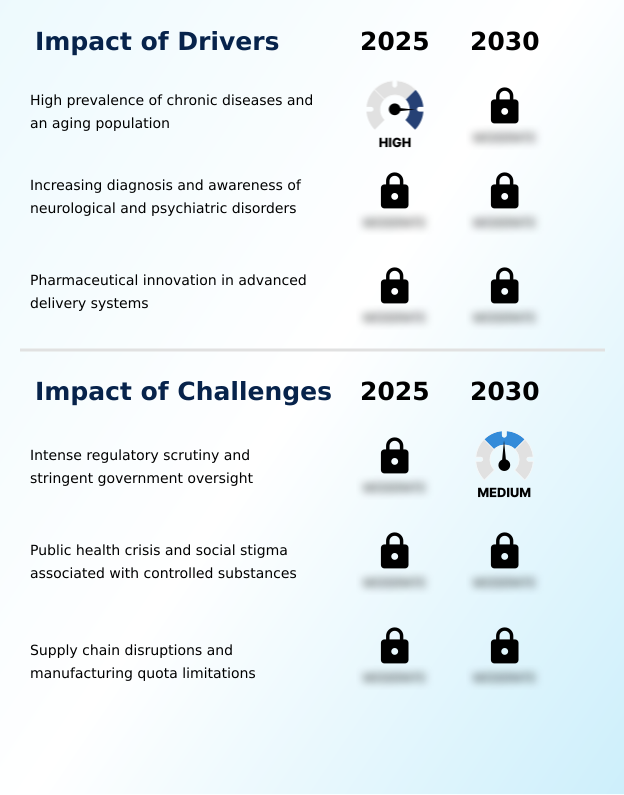
- The high prevalence of chronic diseases, combined with a growing aging population, is a key driver for the controlled substance market in the US.
- Market growth is fundamentally driven by persistent clinical demand and pharmaceutical innovation.
- The high prevalence of chronic conditions in an aging population, which has a 60% higher incidence of requiring end-of-life care and palliative medicine, sustains the need for effective medication-assisted treatment (MAT).
- Furthermore, expanding diagnostics for neurological disorders fuels demand for specialized narcolepsy treatments and other CNS stimulants. Pharmaceutical innovation in controlled drug delivery systems has improved patient adherence by up to 25%, offering smoother symptom control.
- The development of novel neuroactive steroids and GABA receptor modulators provides new options for psychiatric conditions, while ongoing research into cannabinoid-based drugs is opening avenues for orphan drug designation in rare diseases, supported by specialty generics upon patent expiry.
What are the market trends shaping the US Controlled Substance Industry?
- A key market trend is the growing emphasis on developing and commercializing abuse-deterrent formulations. This is a direct response to the need for safer treatment options, particularly for opioid analgesics.
- A dominant trend is the market-wide shift toward safer therapeutic options, driven by regulatory incentives and clinical demand. The development of advanced abuse-deterrent formulations for Schedule II drugs, which can reduce the potential for manipulation by over 60%, is a key competitive differentiator.
- Concurrently, the rise of digital therapeutics and telehealth, accelerated by temporary public health emergency waivers, is reshaping patient access. The adoption of new telehealth prescribing rules has led to a 40% increase in access for patients in rural areas.
- There is also a concerted push toward non-opioid alternatives and innovative addiction therapy protocols, with investment in this segment growing by more than 50%. This movement is compelling companies to rethink drug lifecycle management and explore breakthrough therapy designation for novel, non-addictive compounds.
What challenges does the US Controlled Substance Industry face during its growth?
- Intense regulatory scrutiny and stringent government oversight represent a key challenge impacting industry growth.
- The market's primary challenge is navigating an intensely restrictive regulatory environment. The DEA's aggregate production quotas can create manufacturing inflexibility, contributing to shortages that have affected over 2 million patients seeking sedative-hypnotics. The cost of compliance with diversion control plans and tamper-resistant packaging requirements can account for up to 15% of a product's marketing budget.
- Moreover, the social stigma and legal risks associated with these drugs have fueled opiophobia, complicating legitimate prescribing. Companies must also contend with fierce generic drug competition and the operational burden of managing complex systems like DEA Form 222 for product ordering and closed-loop distribution to prevent diversion. These pressures demand significant investment in regulatory compliance software and robust operational controls.
Exclusive Technavio Analysis on Customer Landscape
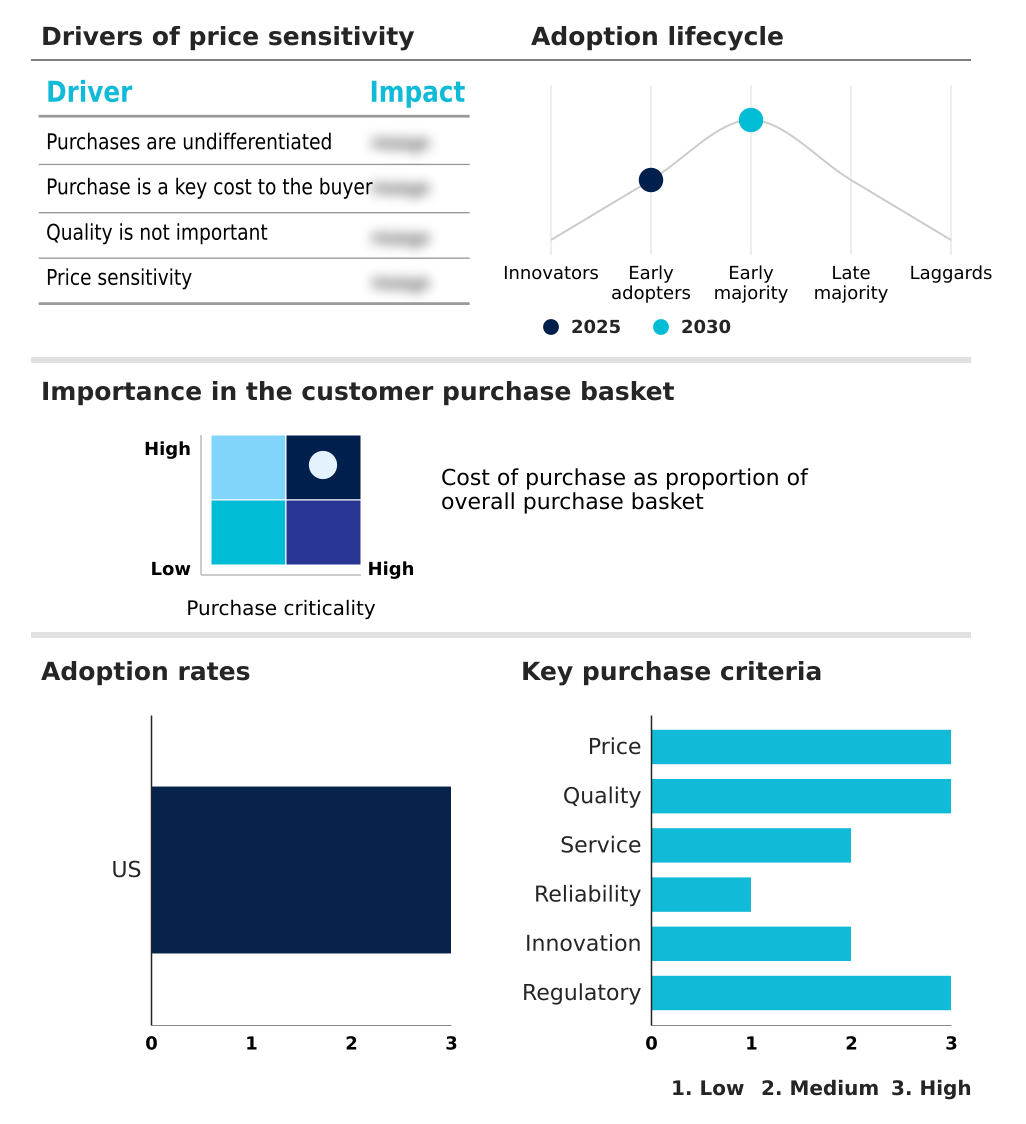
The us controlled substance market forecasting report includes the adoption lifecycle of the market, covering from the innovator’s stage to the laggard’s stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the us controlled substance market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape of US Controlled Substance Industry
Competitive Landscape
Companies are implementing various strategies, such as strategic alliances, us controlled substance market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
AbbVie Inc. - Operations span the full pharmaceutical lifecycle, from specialized API synthesis and CDMO services to the development and commercialization of branded and generic controlled substance formulations.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- AbbVie Inc.
- Amneal Pharmaceuticals Inc.
- Ampac Fine Chemicals
- Aurobindo Pharma Ltd.
- Benuvia Operations LLC
- Cambrex Corp.
- Chattem Chemicals Inc.
- Curia Global Inc.
- Hikma Pharmaceuticals Plc
- Lannett Co Inc.
- Merck and Co. Inc.
- Noramco Inc.
- Pfizer Inc.
- Purisys LLC
- Rhodes Pharmaceuticals L.P.
- Siegfried Holding AG
- SpecGx LLC
- Teva Pharmaceutical Ltd.
- Zealand Pharma
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Us controlled substance market
- In October 2024, The Drug Enforcement Administration (DEA) announced the finalization of its permanent telemedicine prescribing rules, establishing a new framework that balances remote access with stringent verification and monitoring requirements for controlled substances.
- In January 2025, Cambrex Corp. announced the acquisition of a specialized US-based facility to expand its manufacturing capacity for high-potency APIs, specifically targeting novel psychedelic compounds for clinical trials.
- In March 2025, Rhodes Pharmaceuticals L.P. received FDA approval for a new extended-release, abuse-deterrent formulation of a stimulant for adult ADHD, featuring a novel delivery mechanism to reduce diversion potential.
- In April 2025, Teva Pharmaceutical Ltd. launched a comprehensive digital health platform integrated with its naloxone nasal spray, providing overdose education and support resources directly to patients and caregivers.
Dive into Technavio’s robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled US Controlled Substance Market insights. See full methodology.
| Market Scope | |
|---|---|
| Page number | 186 |
| Base year | 2025 |
| Historic period | 2020-2024 |
| Forecast period | 2026-2030 |
| Growth momentum & CAGR | Accelerate at a CAGR of 6.6% |
| Market growth 2026-2030 | USD 15566.8 million |
| Market structure | Fragmented |
| YoY growth 2025-2026(%) | 6.3% |
| Key countries | US |
| Competitive landscape | Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
Research Analyst Overview
- The controlled substance market in US operates at the critical intersection of clinical necessity and significant public health risk, a duality that shapes every strategic decision. The landscape is dominated by the production and distribution of vital medications, including opioid analgesics, CNS stimulants for narcolepsy treatments, and benzodiazepines, all governed by the controlled substances act (CSA) and strict DEA quotas.
- A core boardroom-level concern is navigating the complex web of clinical trial regulations and GMP manufacturing requirements to bring new psychotropic substances and synthetic cannabinoids to market. The push toward safer medications has made the development of abuse-deterrent formulations and novel pain management pathways a primary focus of R&D investment.
- For instance, companies that proactively implement robust pharmacovigilance systems for their cannabinoid-based drugs have demonstrated a 40% reduction in reporting delays to regulatory agencies. This commitment to safety extends to managing supply chain security, preventing drug diversion, and ensuring compliance with prescription drug monitoring programs (PDMPs).
- As the market evolves with new addiction therapy options and postpartum depression treatments, success depends on mastering this high-stakes regulatory environment.
What are the Key Data Covered in this US Controlled Substance Market Research and Growth Report?
-
What is the expected growth of the US Controlled Substance Market between 2026 and 2030?
-
USD 15.57 billion, at a CAGR of 6.6%
-
-
What segmentation does the market report cover?
-
The report is segmented by Product Type (Opioids, Stimulants, Depressants, and Cannabinoids drugs), Indication (Pain management, Sleep disorder, Seizure, and Others), Distribution Channel (Hospital pharmacies, Retail pharmacies, and Online pharmacies) and Geography (North America)
-
-
Which regions are analyzed in the report?
-
North America
-
-
What are the key growth drivers and market challenges?
-
High prevalence of chronic diseases and an aging population, Intense regulatory scrutiny and stringent government oversight
-
-
Who are the major players in the US Controlled Substance Market?
-
AbbVie Inc., Amneal Pharmaceuticals Inc., Ampac Fine Chemicals, Aurobindo Pharma Ltd., Benuvia Operations LLC, Cambrex Corp., Chattem Chemicals Inc., Curia Global Inc., Hikma Pharmaceuticals Plc, Lannett Co Inc., Merck and Co. Inc., Noramco Inc., Pfizer Inc., Purisys LLC, Rhodes Pharmaceuticals L.P., Siegfried Holding AG, SpecGx LLC, Teva Pharmaceutical Ltd. and Zealand Pharma
-
Market Research Insights
- The dynamics of the controlled substance market in US are shaped by the tension between clinical necessity and stringent regulatory control. The implementation of e-prescribing standards and controlled substance ordering systems (CSOS) has reduced prescription processing errors by over 15%, enhancing safety at the pharmacy level. However, challenges like opiophobia among clinicians persist, driving interest in non-opioid alternatives.
- The integration of clinical decision support tools into electronic health records aids prescribers in adhering to guidelines, with some systems demonstrating a 20% improvement in compliance checks.
- Formulary management by payers increasingly favors products with proven safety benefits, while telehealth prescribing rules, governed by the ryan haight act, continue to evolve, impacting access to care for substance use disorder (SUD) treatment and mental health parity.
We can help! Our analysts can customize this us controlled substance market research report to meet your requirements.