Ventricular Drainage Devices Market Size 2024-2028
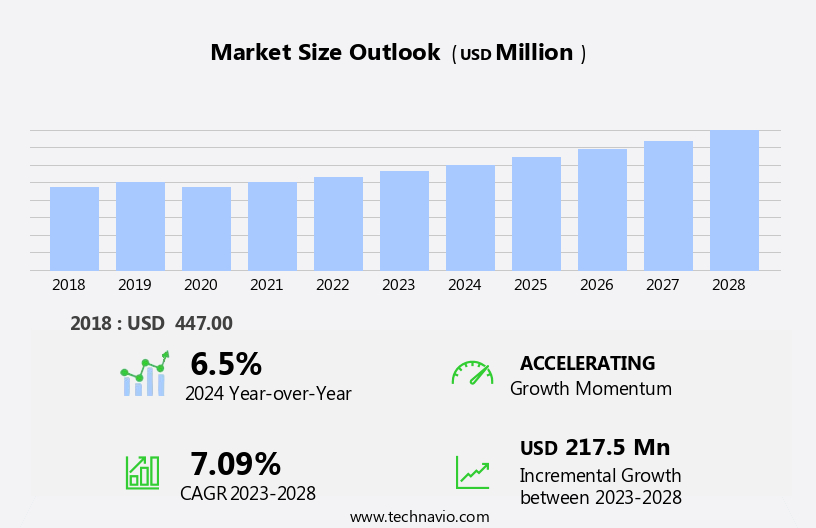
The ventricular drainage devices market size is forecast to increase by USD 217.5 million, at a CAGR of 7.09% between 2023 and 2028.
- The market is experiencing significant growth, driven by the increasing prevalence of brain surgeries and the expansion of neurology specialty hospitals and clinics. Brain surgeries, particularly those involving traumatic injuries or tumors, often necessitate the use of ventricular drainage devices to manage cerebrospinal fluid buildup and prevent complications. This trend is further fueled by the growing number of neurology hospitals and clinics, which cater to the specialized medical needs of neurological patients. However, the market faces challenges, including the high costs associated with brain surgeries and ventricular drainage devices. These expenses can limit access to care for some patients, particularly those without adequate insurance coverage or financial resources.
- Additionally, the development of alternative treatments and technologies, such as endoscopic procedures and minimally invasive devices, may pose a threat to the market's growth. Companies in this market must navigate these challenges by focusing on cost reduction strategies, collaborating with healthcare providers to offer affordable financing options, and investing in research and development to create innovative, cost-effective solutions. By addressing these challenges and capitalizing on the growing demand for ventricular drainage devices, market participants can effectively position themselves for long-term success.
What will be the Size of the Ventricular Drainage Devices Market during the forecast period?
Explore in-depth regional segment analysis with market size data - historical 2018-2022 and forecasts 2024-2028 - in the full report.
Request Free Sample
The market continues to evolve, driven by advancements in technology and growing applications across various sectors. Neurological monitoring, shunt revision surgery, and ventricular access ports are integral components of hydrocephalus treatment, which is a primary application of these devices. CSF flow dynamics play a crucial role in ensuring effective drainage, particularly in managing obstructive hydrocephalus. The ongoing unfolding of market activities includes the development of shunt systems with improved shunt series resistance, valve malfunction detection, and pressure monitoring catheters. Post-operative care and catheter patency are essential considerations, with infection control protocols and drainage flow rate optimization playing key roles. Surgical approaches and implantation techniques are continually evolving, with a focus on minimally invasive procedures and gravitational drainage systems.
Ventricular shunt malfunctions, catheter occlusion, and device longevity remain significant challenges, leading to ongoing research and development efforts. CSF diversion procedures, such as ventriculoperitoneal shunts, offer alternative solutions. Complication management, infection prevention, and fluid pressure regulation are ongoing priorities, with advancements in drainage tube material and subcutaneous reservoirs contributing to improved patient outcomes. Drainage system maintenance and ICP monitoring technology further enhance the effectiveness and safety of these devices.
How is this Ventricular Drainage Devices Industry segmented?
The ventricular drainage devices industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2024-2028, as well as historical data from 2018-2022 for the following segments.
- Product
- Ventricular drainage accessories
- Ventricular drainage systems
- Geography
- North America
- US
- Canada
- Europe
- Germany
- UK
- APAC
- China
- Rest of World (ROW)
- North America
By Product Insights
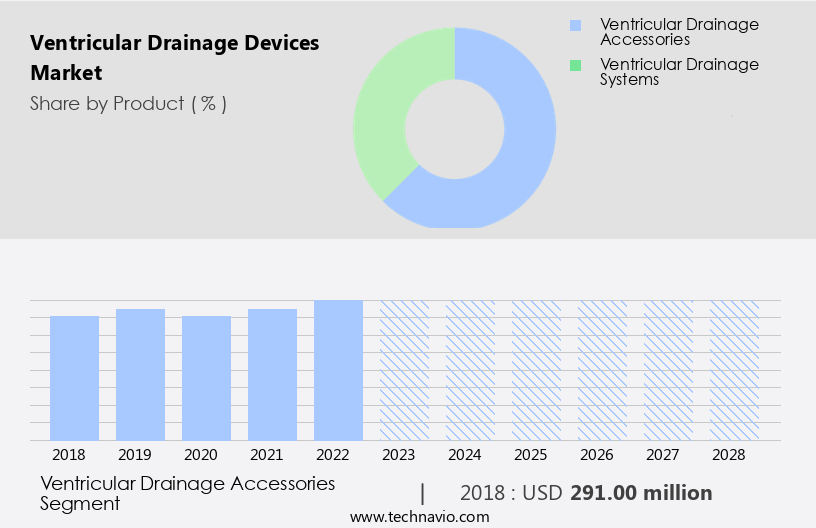
The ventricular drainage accessories segment is estimated to witness significant growth during the forecast period.
Ventricular drainage devices are integral to managing hydrocephalus, a neurological condition characterized by the abnormal accumulation of cerebrospinal fluid (CSF) in the ventricles of the brain. These devices include ventricular access ports, external ventricular drains, and programmable valve systems. The market is witnessing significant advancements in shunt technology, with a focus on improving shunt series resistance for efficient CSF flow dynamics. Neurological monitoring during shunt revision surgery is crucial for detecting valve malfunctions and catheter occlusions, ensuring effective hydrocephalus treatment. Companies are innovating to address drainage device complications, such as catheter-associated infections and infection control. They are developing infection control protocols, pressure monitoring catheters, and infection prevention strategies, including antimicrobial coatings and subcutaneous reservoirs.
Drainage system maintenance is another area of focus, with companies offering drainage tube materials that ensure catheter patency and longevity. Surgical approaches to implantation techniques continue to evolve, with a shift towards minimally invasive procedures and gravitational drainage systems. CSF drainage systems and diversion procedures are also gaining popularity for their effectiveness in managing obstructive hydrocephalus. Valve malfunction detection and programmable valve settings are essential features in ensuring proper fluid pressure regulation and complication management. Ventriculoatrial shunts and ventriculoperitoneal shunts remain the primary treatment options for hydrocephalus. However, the market is witnessing a trend towards advanced shunt systems that offer improved patient outcomes and reduced complications.
Companies are investing in research and development to address the challenges of shunt infection prevention and device longevity.
The Ventricular drainage accessories segment was valued at USD 291.00 million in 2018 and showed a gradual increase during the forecast period.
Regional Analysis
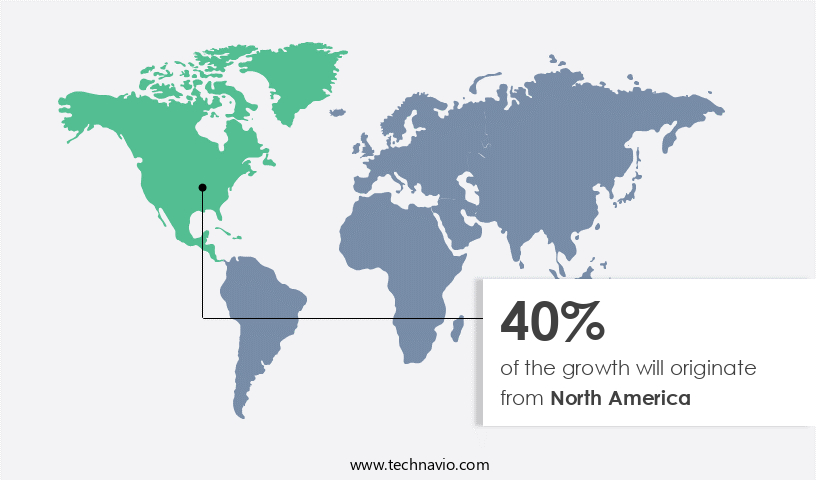
North America is estimated to contribute 40% to the growth of the global market during the forecast period. Technavio's analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
The North American market for ventricular drainage devices is poised for steady expansion, fueled by several factors. The region's growing elderly population, rising incidence of neurological disorders such as traumatic brain injury and brain tumors, and a substantial presence of major industry players are key driving forces. According to the Centers for Disease Control and Prevention (CDC), approximately 795,000 Americans experience a new or recurrent stroke each year, and about 610,000 are diagnosed with a new stroke. The increasing prevalence of conditions like diabetes, obesity, and high cholesterol is expected to further fuel the rising incidence of neurological disorders.
In response to this trend, there is a growing focus on advanced ventricular drainage devices, including those with improved shunt series resistance, neurological monitoring capabilities, and valve malfunction detection systems. These devices aim to enhance patient care, minimize complications, and optimize fluid pressure regulation during shunt revision surgeries and post-operative care. Additionally, there is a growing emphasis on infection control protocols, catheter patency, and drainage system maintenance to mitigate catheter-associated infections. Surgical approaches, such as implantation techniques and gravitational drainage, continue to evolve, as do CSF drainage systems and CSF diversion procedures. The market's longevity and success hinge on effective complication management, including the prevention and treatment of ventricular shunt malfunctions, ventriculoatrial shunts, and shunt infections.
Programmable valve settings and catheter occlusion detection systems are also gaining traction to improve device performance and patient outcomes.
Market Dynamics
Our researchers analyzed the data with 2023 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
What are the key market drivers leading to the rise in the adoption of Ventricular Drainage Devices Industry?
- The rising prevalence of brain surgeries serves as the primary market driver, underpinned by advancements in neurotechnology and growing awareness and acceptance of neurosurgical procedures.
- CSF drainage systems play a crucial role in managing hydrocephalus and other neurological conditions that require cerebrospinal fluid (CSF) diversion procedures. The increasing prevalence of traumatic brain injuries (TBIs) due to various reasons, including chronic conditions and road accidents, drives the demand for these devices. In the US, for instance, about 2 million people are injured in motor vehicle crashes each year, according to the Centers for Disease Control and Prevention (CDC). CSF drainage systems, including ventriculoperitoneal shunts and ventriculoatrial shunts, help manage the buildup of CSF and reduce intracranial pressure. However, these devices come with risks such as infection and complications.
- Therefore, there is a growing focus on shunt infection prevention and complication management. Device longevity is another critical factor influencing the market dynamics. The market is expected to grow as the need for effective CSF drainage systems continues to increase due to the rising incidence of TBIs and other neurological conditions.
What are the market trends shaping the Ventricular Drainage Devices Industry?
- The growing trend in healthcare involves the expansion of neurology specialty hospitals and clinics. This sector is experiencing significant growth, with a heightened focus on providing specialized care for neurological conditions.
- Neurology hospitals and rehabilitation centers are witnessing significant growth due to the rising number of neurological disorders and traumatic brain injuries (TBIs) leading to brain surgeries. Hospitals, governments, and private organizations are investing in advanced care facilities to cater to this increasing patient population. For instance, the Barrow Neurological Institute offers intensive day programs and outpatient rehabilitation services for older adolescents and adults recovering from brain surgeries. Ventricular drainage devices play a crucial role in managing conditions such as obstructive hydrocephalus and intracranial pressure (ICP) disorders. These devices facilitate CSF flow dynamics and enable neurological monitoring through ICP monitoring technology.
- External ventricular drains and ventricular access ports are commonly used drainage devices. Despite their benefits, ventricular drainage devices come with potential complications, including infection, hemorrhage, and shunt series resistance. Shunt revision surgery may be necessary to address these complications. To minimize risks, proper insertion techniques, regular maintenance, and timely intervention are essential. Innovations in ICP monitoring technology and shunt design continue to improve the safety and efficacy of ventricular drainage devices. These advancements aim to reduce complications and enhance patient outcomes.
What challenges does the Ventricular Drainage Devices Industry face during its growth?
- The escalating costs linked to brain surgeries represent a significant barrier to the expansion of the healthcare industry.
- The global market for ventricular drainage devices is driven by the increasing incidence of brain disorders and the subsequent need for advanced treatments, such as hydrocephalus treatment. These devices play a crucial role in post-operative care by facilitating fluid pressure regulation and catheter patency. Pressure monitoring catheters enable real-time detection of valve malfunctions, ensuring effective treatment and reducing the risk of complications. However, the market is challenged by the high costs associated with brain surgeries and the risk of catheter-associated infections.
- The use of advanced materials, such as silicone and polyurethane, in the manufacture of drainage tubes and subcutaneous reservoirs, enhances the durability and functionality of these devices. Despite these challenges, the market is expected to grow due to the increasing demand for minimally invasive procedures and the development of technologically advanced devices.
Exclusive Customer Landscape
The ventricular drainage devices market forecasting report includes the adoption lifecycle of the market, covering from the innovator's stage to the laggard's stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the ventricular drainage devices market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape
Key Companies & Market Insights
Companies are implementing various strategies, such as strategic alliances, ventricular drainage devices market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
Acandis GmbH - This company specializes in manufacturing ventricular drainage devices, designed for implantation to facilitate the removal of excessive cerebrospinal fluid to alternative body locations. These medical devices enhance patient recovery by alleviating intracranial pressure.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Acandis GmbH
- B.Braun SE
- BIOMEDICA ITALIA S.R.L.
- Calon Cardio Technology Ltd.
- Delta
- Desu Medical
- Fuji Systems Co. Ltd.
- Integra Lifesciences Corp.
- IRRAS AB
- LivaNova PLC
- Medtronic Plc
- Moller Medical GmbH
- Natus Medical Inc.
- Neuromedex GmbH
- Salvavidas Pharmaceutical Pvt. Ltd.
- Silmag
- Sophysa
- Spiegelberg GmbH and Co. KG
- Wellona Pharma
- Yushin Medical Co. Ltd.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Ventricular Drainage Devices Market
- In January 2024, Medtronic plc, a global healthcare solutions company, announced the FDA approval of its IntellaSite Model 7100 Ventricular Drainage System. This advanced system utilizes real-time CSF pressure monitoring to optimize drainage and reduce complications (Medtronic Press Release, 2024).
- In March 2024, Stryker Corporation entered into a strategic partnership with Integra LifeSciences to expand its neuro and spine portfolio. The collaboration included the acquisition of Integra's neuro and spine business, which included ventricular drainage devices, enhancing Stryker's presence in the neuro market (Stryker Press Release, 2024).
- In April 2025, Covidien plc, a global healthcare leader, received CE Mark approval for its new Hydrocephalus Shunt System, which includes a programmable ventricular drainage component. This system offers remote programming capabilities, improving patient care and reducing hospital visits (Covidien Press Release, 2025).
- In May 2025, Smith & Nephew plc, a leading medical device manufacturer, announced the acquisition of ArthroCare Corporation's Neuro and Spine business. This acquisition included the Neuro2 ProGAVe Ventricular Drainage System, expanding Smith & Nephew's neuro and spine portfolio (Smith & Nephew Press Release, 2025).
Research Analyst Overview
- The market encompasses innovative designs in drainage system architecture, focusing on durability and effective CSF pressure control. Shunt valve mechanisms continue to evolve, enhancing neurological recovery and surgical outcomes. Drainage tube diameter and material properties are crucial factors in shunt failure diagnosis and long-term outcomes. Icp waveform analysis and diagnostic imaging aid in assessing shunt performance and infection risk factors. Complication rates, including tissue response and implantation site selection, are under constant scrutiny.
- Clinical trials and patient selection criteria are essential for evaluating treatment efficacy and system reliability. Valve durability and device biocompatibility remain key considerations in hydrocephalus management. Maintenance protocols and device safety are paramount in ensuring quality of life for patients.
Dive into Technavio's robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Ventricular Drainage Devices Market insights. See full methodology.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
138 |
|
Base year |
2023 |
|
Historic period |
2018-2022 |
|
Forecast period |
2024-2028 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 7.09% |
|
Market growth 2024-2028 |
USD 217.5 million |
|
Market structure |
Fragmented |
|
YoY growth 2023-2024(%) |
6.5 |
|
Key countries |
US, Germany, China, Canada, and UK |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
What are the Key Data Covered in this Ventricular Drainage Devices Market Research and Growth Report?
- CAGR of the Ventricular Drainage Devices industry during the forecast period
- Detailed information on factors that will drive the growth and forecasting between 2024 and 2028
- Precise estimation of the size of the market and its contribution of the industry in focus to the parent market
- Accurate predictions about upcoming growth and trends and changes in consumer behaviour
- Growth of the market across North America, Europe, Asia, and Rest of World (ROW)
- Thorough analysis of the market's competitive landscape and detailed information about companies
- Comprehensive analysis of factors that will challenge the ventricular drainage devices market growth of industry companies
We can help! Our analysts can customize this ventricular drainage devices market research report to meet your requirements.