Viral Vector Manufacturing Market Size 2025-2029
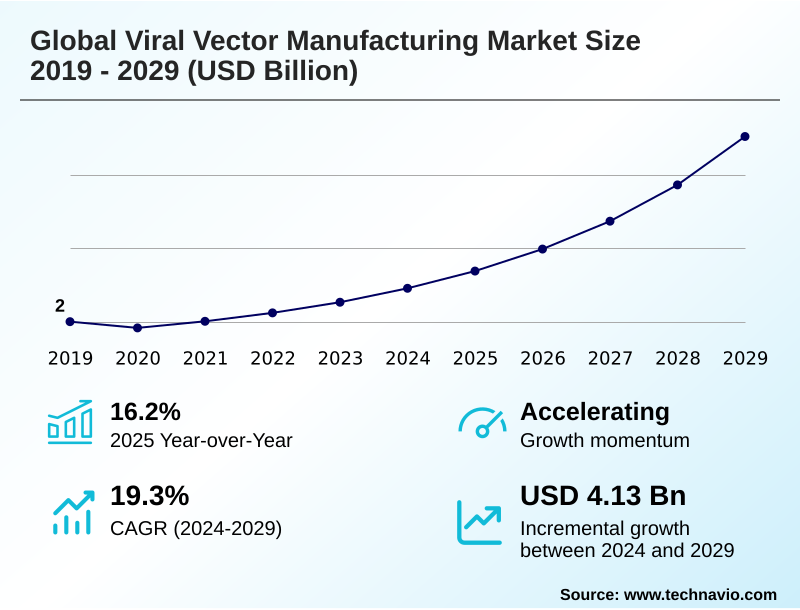
The viral vector manufacturing market size is valued to increase by USD 4.13 billion, at a CAGR of 19.3% from 2024 to 2029. Expanding pipeline and regulatory approval of cell and gene therapies will drive the viral vector manufacturing market.
Major Market Trends & Insights
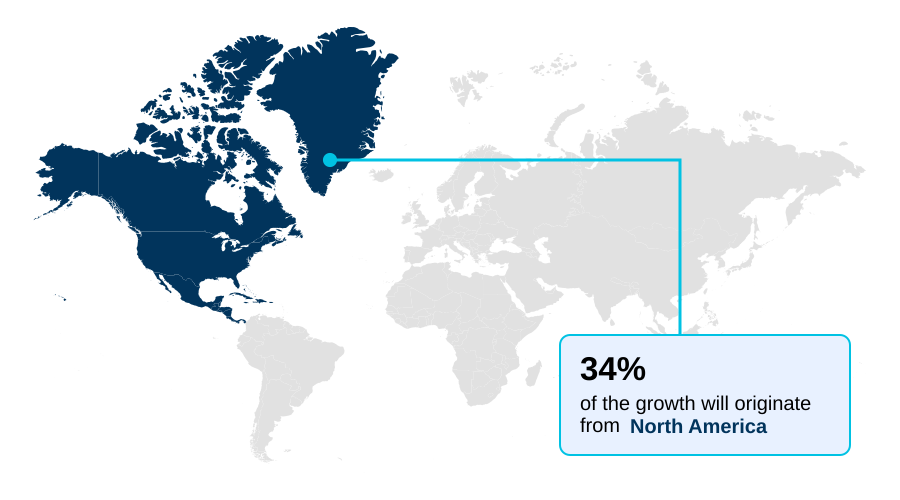
- North America dominated the market and accounted for a 33.6% growth during the forecast period.
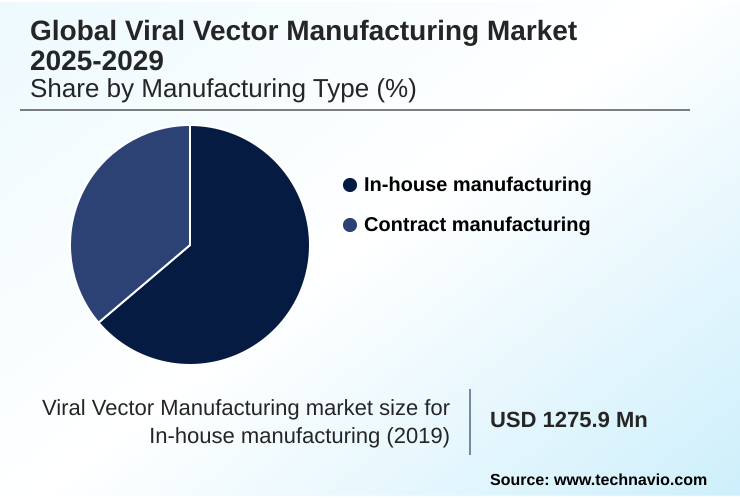
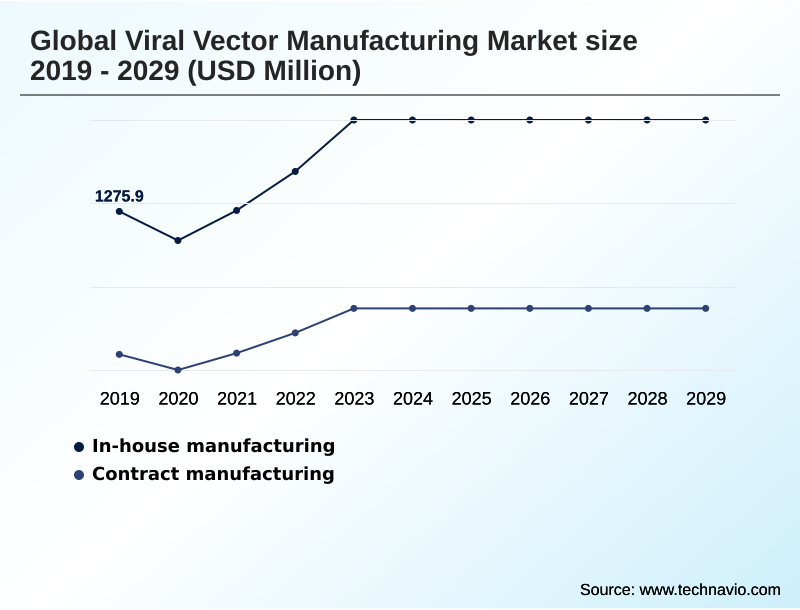
- By Manufacturing Type - In-house manufacturing segment was valued at USD 1.63 billion in 2023
- By Application - Genetic disorders segment accounted for the largest market revenue share in 2023
Market Size & Forecast
- Market Opportunities: USD 5.05 billion
- Market Future Opportunities: USD 4.13 billion
- CAGR from 2024 to 2029 : 19.3%
Market Summary
- The viral vector manufacturing market is integral to the advancement of modern medicine, providing the critical delivery mechanisms for transformative treatments. This sector focuses on the development and production of engineered viruses, such as adeno-associated virus vectors and lentiviral vectors, designed to deliver a therapeutic payload delivery to target cells.
- The primary applications are in cell and gene therapies, including life-saving CAR-T cell therapies for cancer and in vivo gene therapies for genetic disorders. Key industry drivers include an expanding pipeline of regenerative medicine applications and increasing regulatory approvals, which create sustained demand for clinical-grade vector supply and commercial-scale manufacturing. However, the industry grapples with significant challenges.
- For example, a biopharmaceutical firm aiming to scale up production must navigate complex downstream purification process hurdles, such as separating empty versus full capsids, which directly impacts product potency and safety. This requires sophisticated viral vector characterization and adherence to stringent cGMP manufacturing compliance.
- The continuous evolution of gene editing technologies and viral vector platforms further drives the need for innovation in scalable manufacturing processes and process intensification strategies to improve vector production yields and reduce costs.
What will be the Size of the Viral Vector Manufacturing Market during the forecast period?
Get Key Insights on Market Forecast (PDF) Request Free Sample
How is the Viral Vector Manufacturing Market Segmented?
The viral vector manufacturing industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2025-2029, as well as historical data from 2019-2023 for the following segments.
- Manufacturing type
- In-house manufacturing
- Contract manufacturing
- Application
- Genetic disorders
- Infectious disease
- Cancer
- Neurological disorders
- Others
- Type
- Adeno-associated viral (AAV) vectors
- Lentiviral vectors
- Adenoviral vectors
- Others
- Geography
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Asia
- Rest of World (ROW)
- North America
By Manufacturing Type Insights
The in-house manufacturing segment is estimated to witness significant growth during the forecast period.
The in-house manufacturing segment is expanding as companies seek greater control over clinical-grade vector supply and the overall drug substance manufacturing process. This strategic pivot allows for direct oversight of cGMP manufacturing compliance and protects proprietary process intensification strategies.
By internalizing production, firms can better manage the entire upstream processing workflow and mitigate supply chain bottlenecks that can delay clinical programs.
This approach also integrates cell line development services more closely with R&D, creating a feedback loop that accelerates bioprocess optimization.
The ability to control the technology transfer protocols internally has been shown to improve project timelines by over 15%, ensuring that advanced therapy medicinal products reach patients faster.
This model provides superior management of raw material qualification and the entire production timeline for advanced biologics.
The In-house manufacturing segment was valued at USD 1.63 billion in 2023 and showed a gradual increase during the forecast period.
Regional Analysis
North America is estimated to contribute 33.6% to the growth of the global market during the forecast period.Technavio’s analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
See How Viral Vector Manufacturing Market Demand is Rising in North America Request Free Sample
North America dominates the market, housing the highest concentration of companies focused on gene therapy delivery systems. However, the APAC region is the fastest-growing market, with government initiatives in countries like China and South Korea fueling investment in biopharmaceutical manufacturing.
This has led to the rise of regional CDMOs capable of handling complex lentiviral vectors and other modalities. European hubs remain critical for plasmid DNA production and specialized cell culture systems, supporting a robust network for commercial drug supply.
The focus on regionalizing the supply chain has become a key strategy, with some Asian facilities demonstrating a 20% lower operational cost structure for certain processes.
This geographic diversification helps manage risks related to vector shedding analysis and ensures a more resilient supply of drug product formulation for the global market, alongside a deeper pool for raw material sourcing.
Market Dynamics
Our researchers analyzed the data with 2024 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
- The strategic decisions facing biopharmaceutical companies today revolve around complex trade-offs, such as outsourcing versus in-house vector manufacturing. While outsourcing to a CDMO can accelerate timelines, internalizing production offers greater control over intellectual property and the supply chain.
- The cost analysis of GMP vector production is a critical factor, with expenses for plasmid DNA as a critical material and purification media contributing significantly to the final cost of goods for gene therapies. For companies focused on oncology, the specifics of viral vector manufacturing for cancer therapy, especially producing lentiviral vector production for CAR-T, present unique challenges.
- The industry is intensely focused on solving technical hurdles, including challenges in scaling up AAV production and finding effective methods for reducing empty capsid ratio. These efforts directly influence improving viral vector purification yields. Simultaneously, navigating regulatory hurdles in viral vector production requires sophisticated analytical methods for vector potency testing and robust quality control testing for viral vectors.
- The impact of automation on vector manufacturing is seen as a key enabler for consistency and efficiency. As companies mature, their process development for viral vectors becomes more sophisticated, incorporating data-driven approaches. The CDMO selection criteria for viral vectors now heavily weighs a partner's technological platform and regulatory track record.
- While adeno-associated vs lentiviral vector manufacturing debates continue based on application, the entire ecosystem is also watching the development of non-viral gene delivery systems.
- Effective supply chain optimization for CGTs is now a core competency, with integrated planning reducing material lead times by over 15% compared to siloed approaches, directly impacting the ability to meet clinical and commercial demand for everything from genetic disorders to viral vector platforms for vaccine development.
What are the key market drivers leading to the rise in the adoption of Viral Vector Manufacturing Industry?
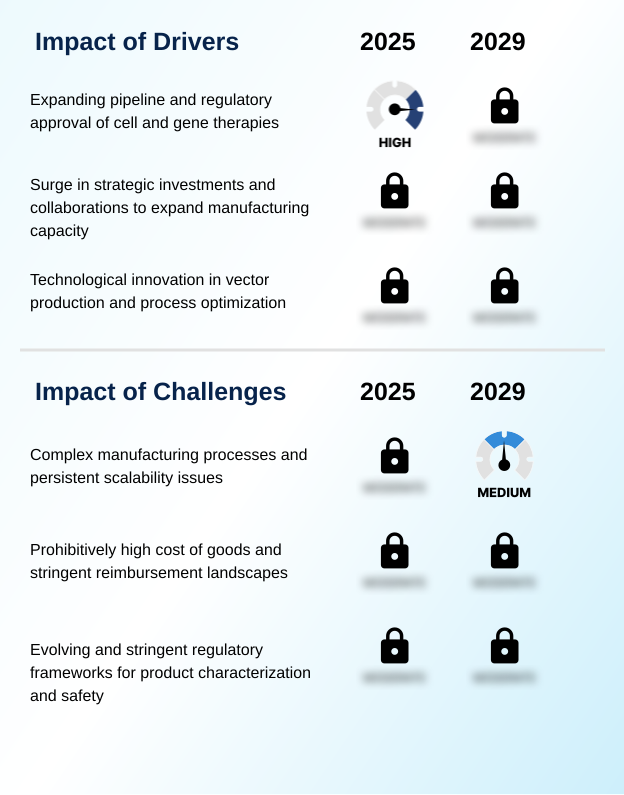
- The expanding clinical pipeline and increasing regulatory approval of cell and gene therapies are the primary drivers for the market.
- The primary driver is the expanding pipeline of cell and gene therapies, particularly for in vivo gene therapies and ex vivo gene modification. Landmark approvals for CAR-T cell therapies and treatments for genetic disorders create tangible demand for commercial-scale manufacturing.
- This has prompted a surge in manufacturing capacity expansion to address the need for high-titer vector production. For instance, facilities are being designed to handle a 50% increase in production volume.
- This growth is supported by investments aimed at de-risking the supply of clinical trial material.
- As more regenerative medicine applications advance, the demand for sophisticated gene editing technologies and their associated therapeutic payload delivery mechanisms intensifies, compelling CDMOs to enhance their fill-finish services.
What are the market trends shaping the Viral Vector Manufacturing Industry?
- A strategic shift toward in-house manufacturing and sophisticated hybrid operational models is an important market trend. This rebalancing is driven by the need for greater supply chain control and long-term cost optimization.
- A key trend is the move toward scalable manufacturing processes that leverage process analytical technology for real-time monitoring. This shift away from traditional batch methods improves vector production yields and ensures greater batch-to-batch consistency, a critical factor for regulatory CMC requirements. Adopting single-use bioreactor systems has been shown to reduce cleaning validation times by over 40%, accelerating production timelines.
- The industry is also diversifying beyond adeno-associated virus vectors to explore oncolytic virus platforms and other viral vector platforms. This requires flexible scalable production platforms and robust analytical method development to handle varied viral vector quality attributes and support immunotherapy development.
What challenges does the Viral Vector Manufacturing Industry face during its growth?
- Complex manufacturing processes and persistent scalability issues present a key challenge affecting industry growth.
- A persistent challenge is the complexity of the downstream purification process, especially in achieving efficient separation of empty versus full capsids. This issue directly impacts therapeutic product efficacy and requires extensive viral clearance validation.
- The high cost of goods is another major hurdle, driven by expensive transient transfection methods and the need for stringent quality control release testing, where costs can represent up to 60% of total production expenses. Navigating aseptic processing standards and the intricacies of viral vector characterization add to the complexity.
- The development of stable producer cell lines and non-viral delivery technologies are promising solutions, but their widespread adoption is hampered by the need for extensive process validation and comparability studies, which can be resource-intensive.
Exclusive Technavio Analysis on Customer Landscape
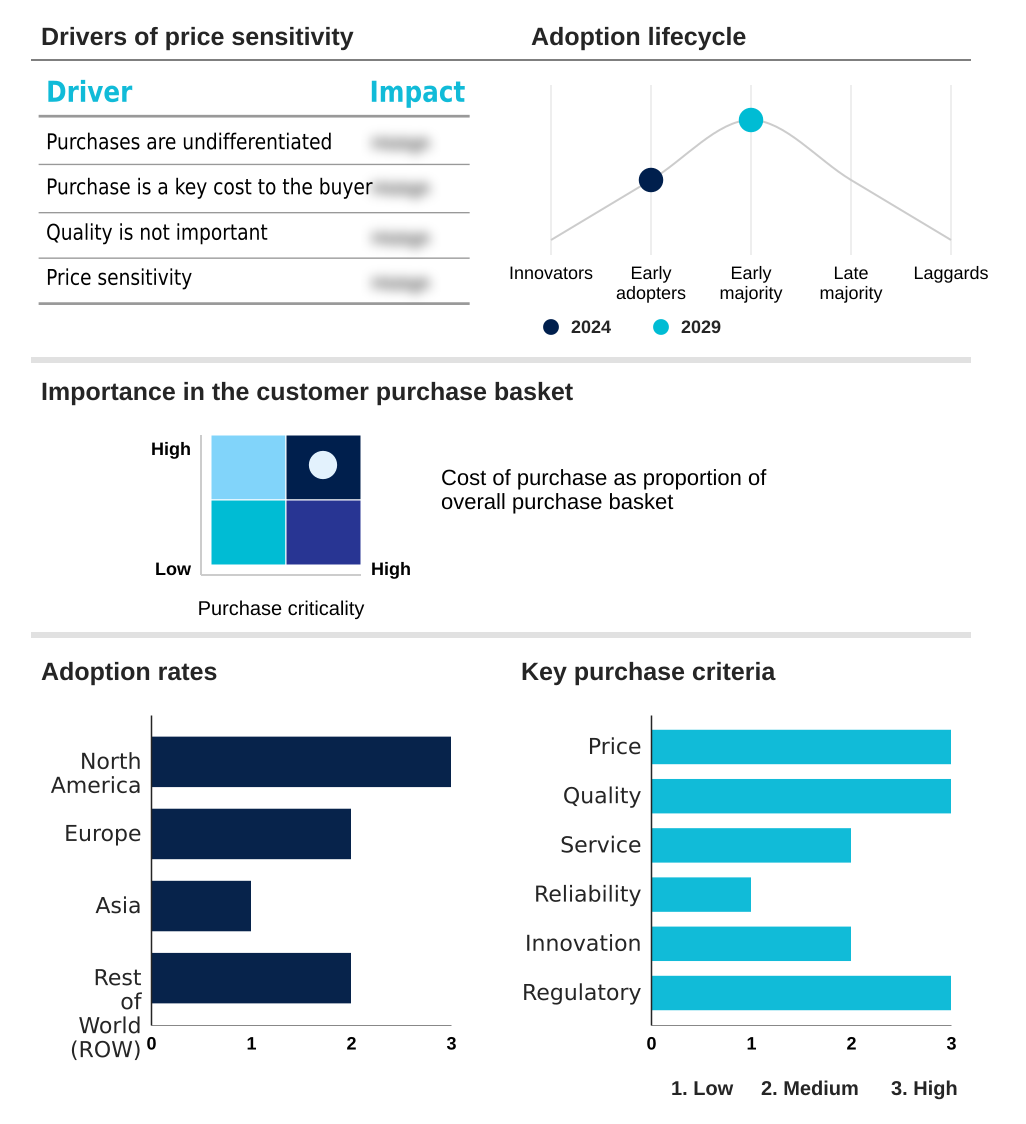
The viral vector manufacturing market forecasting report includes the adoption lifecycle of the market, covering from the innovator’s stage to the laggard’s stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the viral vector manufacturing market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape of Viral Vector Manufacturing Industry
Competitive Landscape
Companies are implementing various strategies, such as strategic alliances, viral vector manufacturing market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
AGC Biologics - Offerings include end-to-end development and cGMP manufacturing of viral vectors, plasmids, and advanced biologics for cell and gene therapy applications, from preclinical to commercial stages.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- AGC Biologics
- Aldevron LLC
- Andelyn Biosciences
- Batavia Biosciences BV
- Catalent Inc.
- Charles River Laboratories
- Exothera
- FUJIFILM Holdings Corp.
- Genezen
- Lonza Group Ltd.
- Miltenyi Biotec
- Oxford Biomedica Plc
- Sigma Aldrich Chemicals Ltd.
- SK Pharmteco
- Takara Bio Inc.
- Thermo Fisher Scientific Inc.
- Virovek Inc
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Viral vector manufacturing market
- In August, 2024, BioGenix Solutions announced the opening of a 250,000-square-foot facility dedicated to in-house commercial-scale manufacturing of its AAV-based gene therapy portfolio, reducing its reliance on CDMOs.
- In November, 2024, VectorFlow CDMO completed its acquisition of Plasmid Technologies Inc. for USD 450 million, securing a critical part of the supply chain for viral vector starting materials.
- In February, 2025, OmniProcess Innovators launched its new automated 'VectorPro' platform, which integrates upstream and downstream processing to reduce manual intervention and improve batch consistency by up to 25%.
- In May, 2025, CureGene Therapeutics received regulatory approval for its lentiviral vector-based CAR-T therapy for late-stage lymphoma, triggering significant demand for commercial-grade vector supply.
Dive into Technavio’s robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Viral Vector Manufacturing Market insights. See full methodology.
| Market Scope | |
|---|---|
| Page number | 301 |
| Base year | 2024 |
| Historic period | 2019-2023 |
| Forecast period | 2025-2029 |
| Growth momentum & CAGR | Accelerate at a CAGR of 19.3% |
| Market growth 2025-2029 | USD 4134.3 million |
| Market structure | Fragmented |
| YoY growth 2024-2025(%) | 16.2% |
| Key countries | US, Canada, Mexico, Germany, UK, France, Italy, The Netherlands, Spain, Russia, China, Japan, India, South Korea, Indonesia, Thailand, Singapore, Australia, UAE, Brazil, South Africa, Saudi Arabia and Turkey |
| Competitive landscape | Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
Research Analyst Overview
- The viral vector manufacturing market is undergoing a significant industrialization phase, moving beyond academic-scale methods toward robust, commercial-scale manufacturing. This evolution is driven by the demand for cell and gene therapies, necessitating advanced viral vector platforms capable of producing high-quality adeno-associated virus vectors and lentiviral vectors.
- A central boardroom focus is mitigating manufacturing risk through strategies like developing stable producer cell lines to replace less consistent transient transfection methods. This pivot improves vector production yields and enhances process control. The complexity of the downstream purification process, particularly managing the ratio of empty versus full capsids, remains a critical challenge impacting both cost and quality.
- Firms that master viral vector characterization and process analytical technology are gaining a competitive edge. For instance, implementing PAT has enabled some manufacturers to reduce out-of-spec batches by over 40%, directly impacting bottom-line profitability.
- Success in this market depends on mastering cGMP manufacturing compliance while advancing process intensification strategies and scalable manufacturing processes for both in vivo gene therapies and ex vivo gene modification. The development of non-viral delivery technologies also looms as a future disruptive force.
What are the Key Data Covered in this Viral Vector Manufacturing Market Research and Growth Report?
-
What is the expected growth of the Viral Vector Manufacturing Market between 2025 and 2029?
-
USD 4.13 billion, at a CAGR of 19.3%
-
-
What segmentation does the market report cover?
-
The report is segmented by Manufacturing Type (In-house manufacturing, and Contract manufacturing), Application (Genetic disorders, Infectious disease, Cancer, Neurological disorders, and Others), Type (Adeno-associated viral (AAV) vectors, Lentiviral vectors, Adenoviral vectors, and Others) and Geography (North America, Europe, Asia, Rest of World (ROW))
-
-
Which regions are analyzed in the report?
-
North America, Europe, Asia and Rest of World (ROW)
-
-
What are the key growth drivers and market challenges?
-
Expanding pipeline and regulatory approval of cell and gene therapies, Complex manufacturing processes and persistent scalability issues
-
-
Who are the major players in the Viral Vector Manufacturing Market?
-
AGC Biologics, Aldevron LLC, Andelyn Biosciences, Batavia Biosciences BV, Catalent Inc., Charles River Laboratories, Exothera, FUJIFILM Holdings Corp., Genezen, Lonza Group Ltd., Miltenyi Biotec, Oxford Biomedica Plc, Sigma Aldrich Chemicals Ltd., SK Pharmteco, Takara Bio Inc., Thermo Fisher Scientific Inc. and Virovek Inc
-
Market Research Insights
- The market's dynamism is shaped by the imperative to improve efficiency and reduce costs. Organizations are achieving greater batch-to-batch consistency through advanced bioprocess optimization, with leading CDMOs reporting up to a 30% reduction in process deviations. The focus on cost of goods reduction is intense, as it directly impacts the commercial viability of multi-million dollar therapies.
- The reliance on a stable supply of critical starting materials necessitates robust raw material qualification and sourcing strategies, mitigating supply chain bottlenecks that can delay timelines. Furthermore, the complexity of technology transfer protocols between therapy developers and manufacturing partners is a critical variable; streamlined protocols have been shown to shorten project initiation times by over 25%.
- This push for operational excellence is essential for managing the intricate demands of drug substance manufacturing and ensuring the timely delivery of these advanced treatments.
We can help! Our analysts can customize this viral vector manufacturing market research report to meet your requirements.