Medical Device Manufacturing Outsourcing Market Size 2025-2029
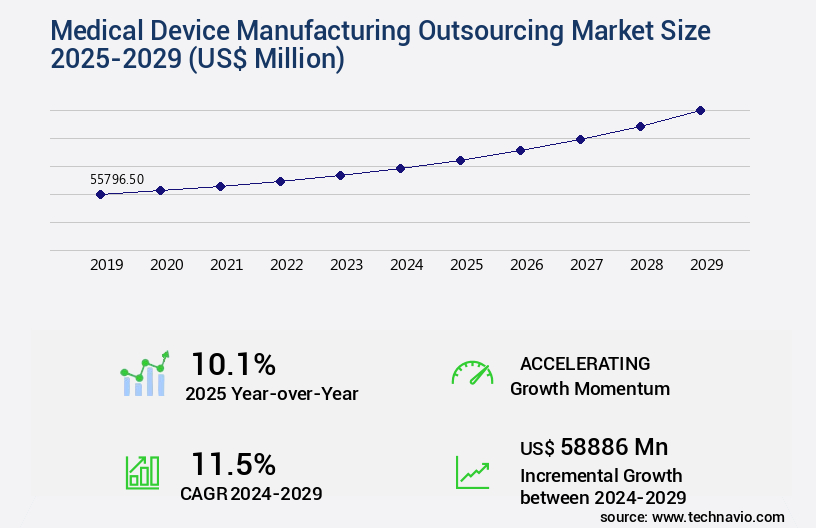
The medical device manufacturing outsourcing market size is valued to increase USD 58.89 billion, at a CAGR of 11.5% from 2024 to 2029. Growing focus of OEMs on reducing medical device manufacturing costs will drive the medical device manufacturing outsourcing market.
Major Market Trends & Insights
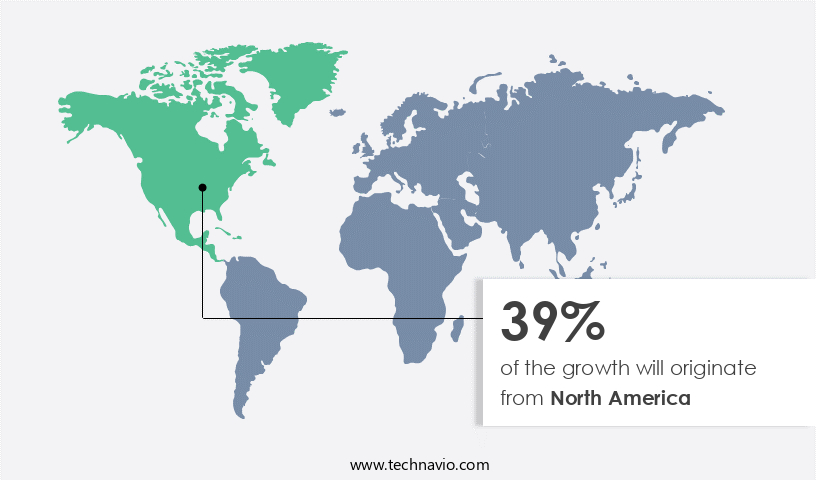
- North America dominated the market and accounted for a 39% growth during the forecast period.
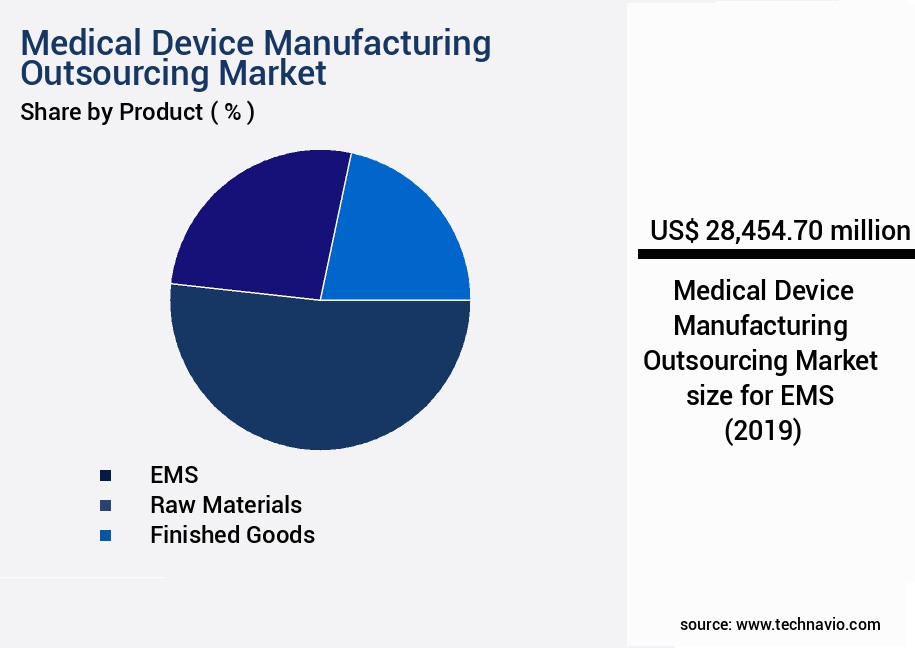
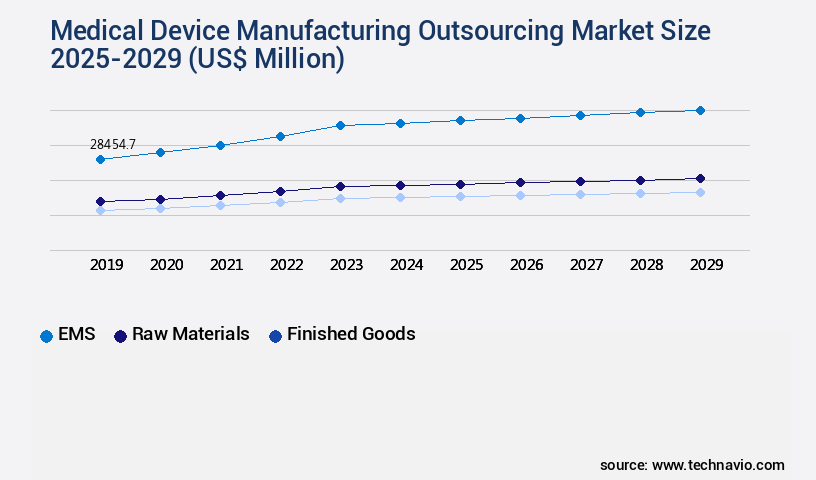
- By Product - EMS segment was valued at USD 28.45 billion in 2023
- By Class Type - Class II segment accounted for the largest market revenue share in 2023
Market Size & Forecast
- Market Opportunities: USD 152.21 million
- Market Future Opportunities: USD 58886.00 million
- CAGR : 11.5%
- North America: Largest market in 2023
Market Summary
- The market represents a significant and continuously evolving sector, with core technologies and applications driving innovation and growth. This market encompasses various service types and product categories, including contract manufacturing, design and development, and testing and certification services. The industry is experiencing a growing focus among Original Equipment Manufacturers (OEMs) to reduce manufacturing costs, leading to increased outsourcing activities. Furthermore, the market is witnessing a surge in mergers and acquisitions, as companies seek to expand their offerings and enhance their competitive positions.
- However, the stringent regulatory environment poses a significant challenge, with regulatory compliance being a critical factor for market participants. According to a recent report, the contract manufacturing segment is expected to account for over 60% of the market share, underscoring its dominance in the industry.
What will be the Size of the Medical Device Manufacturing Outsourcing Market during the forecast period?
Get Key Insights on Market Forecast (PDF) Request Free Sample
How is the Medical Device Manufacturing Outsourcing Market Segmented and what are the key trends of market segmentation?
The medical device manufacturing outsourcing industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2025-2029, as well as historical data from 2019-2023 for the following segments.
- Product
- EMS
- Raw materials
- Finished goods
- Class Type
- Class II
- Class III
- Class I
- Service Type
- Manufacturing services
- Quality assurance and testing
- Device development and design services
- Regulatory and certification support
- End-user
- Medical device companies
- Pharmaceutical and biotech firms
- Hospitals and clinics
- Research institutions
- Geography
- North America
- US
- Canada
- Europe
- France
- Germany
- UK
- APAC
- China
- India
- Japan
- South Korea
- South America
- Brazil
- Rest of World (ROW)
- North America
By Product Insights
The ems segment is estimated to witness significant growth during the forecast period.
In the medical device manufacturing sector, quality assurance plays a pivotal role in ensuring the production of safe and effective devices. Sterilization validation, inventory management, and change control management are crucial processes that contribute to maintaining high product quality. Computer system validation, manufacturing execution systems, design verification, material handling, sterile manufacturing, process capability analysis, and component sourcing are essential elements of the manufacturing process. Regulatory compliance, including quality management systems, cleanroom design, and product lifecycle management, is a significant focus for medical device manufacturers. Equipment qualification, risk management, production yield, and process validation are essential for efficient manufacturing.
Medical device assembly, design control, and production planning are integral parts of the supply chain management strategy. The market is experiencing significant growth, with an estimated 25% of medical devices being outsourced for manufacturing. Furthermore, the market is projected to expand by 27% in the coming years, driven by the increasing demand for cost-effective solutions and the need for specialized expertise. Moreover, the adoption of advanced technologies, such as automation, robotics, and 3D printing, is transforming the medical device manufacturing landscape. These technologies enable manufacturers to improve production efficiency, reduce costs, and enhance product quality. Statistical process control, device testing, and packaging processes are essential components of the manufacturing process that ensure the production of high-quality medical devices.
Contract manufacturing and quality control are also critical aspects of the outsourcing market, enabling OEMs to focus on their core competencies while outsourcing manufacturing to specialized partners. Good manufacturing practices and design control are essential for ensuring regulatory compliance and maintaining product quality. The market is expected to continue evolving, with a focus on innovation, efficiency, and cost savings.
The EMS segment was valued at USD 28.45 billion in 2019 and showed a gradual increase during the forecast period.
Regional Analysis
North America is estimated to contribute 39% to the growth of the global market during the forecast period.Technavio’s analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
See How Medical Device Manufacturing Outsourcing Market Demand is Rising in North America Request Free Sample
The North American the market is experiencing growth, driven by rising research and development investments, mergers and acquisitions, and technological advances. With increasing competition, small and medium-sized companies in sectors like stents, wound care, and orthopedics outsource manufacturing due to limited technological resources and larger capabilities. Major players include those based in New York, Pennsylvania, Michigan, Massachusetts, Illinois, and Minnesota. Market dynamics include collaboration between companies and the maturity of product categories.
Recent statistics indicate a significant increase in outsourcing contracts, a growing number of medical device companies, and a surge in R&D spending. These trends are expected to continue, making the market a significant contributor to the North American economy.
Market Dynamics
Our researchers analyzed the data with 2024 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
The market is a dynamic and intricate ecosystem that encompasses various aspects of medical device production, from component sourcing to final assembly and packaging. This market is characterized by a growing emphasis on strategies that optimize production, ensure regulatory compliance, and maintain the highest levels of quality. Medical device component sourcing strategies have evolved significantly, with an increasing preference for offshore manufacturing to reduce costs and improve efficiency. Cleanroom design for medical device manufacturing plays a crucial role in maintaining the required level of sterility and reducing contamination risks. In this context, sterilization validation techniques are essential to ensure the effectiveness of sterilization processes and maintain product safety.
Process validation and quality management system implementation are integral components of medical device manufacturing, ensuring consistent product quality and adherence to regulatory requirements. Regulatory compliance strategies and risk management techniques are essential to mitigate risks and ensure the safety and efficacy of medical devices. Design control processes and medical device assembly automation techniques are critical to streamline production and reduce errors. Supply chain management and production planning optimization are essential to ensure timely delivery of components and finished products. Failure mode effects analysis and statistical process control are crucial for identifying potential issues and addressing them proactively. Medical device packaging processes and validation are essential to maintain product integrity and ensure safe transportation and storage.
Computer system validation is a critical aspect of medical device manufacturing, ensuring the reliability and security of data and systems. Good manufacturing practices implementation is a prerequisite for market entry and maintaining market presence. Adoption rates of automation and digital technologies in medical device manufacturing are significantly higher than in traditional manufacturing industries. For instance, more than 80% of medical device manufacturers have adopted automation in their production processes, compared to less than 50% in the automotive industry. This trend is expected to continue, with a growing focus on digitalization and data-driven manufacturing.
What are the key market drivers leading to the rise in the adoption of Medical Device Manufacturing Outsourcing Industry?
- The increasing priority of Original Equipment Manufacturers (OEMs) to decrease medical device manufacturing costs serves as the primary market driver.
- In the current economic climate, healthcare budget constraints have intensified the need for medical device manufacturers to deliver cost-effective, high-quality healthcare solutions. To address this challenge, OEMs are increasingly outsourcing medical device manufacturing. The high engineering demands and escalating labor costs associated with producing medical devices make outsourcing an attractive option for cost savings. By outsourcing, OEMs can significantly reduce product development expenses. The burgeoning demand for medical devices and the high potential for growth in this sector have further encouraged OEMs to outsource manufacturing capabilities.
- Depending on the specific company and product, outsourcing can lead to savings of up to 15% of total production costs. This trend is a response to the evolving market landscape and the ongoing need to optimize costs while maintaining quality and innovation.
What are the market trends shaping the Medical Device Manufacturing Outsourcing Industry?
- The upcoming market trend involves an increase in mergers and acquisitions activities. This trend is mandatory for businesses seeking growth and expansion.
- The market is characterized by its high level of competition and fragmentation, with a multitude of global, regional, and local players. In response to this intense competition, mergers and acquisitions have emerged as a popular strategy for inorganic growth. This trend is evident in the increasing number of transactions occurring in the market. Buyers pursue mergers and acquisitions to expand their product offerings, enter new geographies, and strengthen their market position in existing and emerging markets.
- The result is a significant increase in the inorganic growth of leading companies, particularly among larger players. This dynamic market landscape continues to evolve, with ongoing activity and shifting patterns shaping the competitive landscape.
What challenges does the Medical Device Manufacturing Outsourcing Industry face during its growth?
- In the highly regulated business landscape, strict regulatory compliance poses a significant challenge to the industry's growth trajectory.
- Medical device outsourcing market regulations play a pivotal role in shaping the industry's dynamics. With increasing globalization, many Original Equipment Manufacturers (OEMs) opt for outsourcing medical device manufacturing overseas. Regulations in various countries, such as the US and Europe, ensure quality control and environmental compliance. The US Food and Drug Administration (FDA) and the European Commission have implemented stringent regulations for medical device manufacturers, including those providing outsourcing services. For instance, the US FDA's 510(k) process for Class III medical devices involves a more extended approval timeline compared to other regions.
- This regulatory scrutiny ensures the production of safe and effective medical devices. The evolving nature of these regulations necessitates continuous adaptation by outsourcing service providers to maintain compliance and meet market demands. By adhering to these regulations, OEMs can ensure the production of high-quality medical devices while minimizing risks associated with regulatory non-compliance.
Exclusive Customer Landscape
The medical device manufacturing outsourcing market forecasting report includes the adoption lifecycle of the market, covering from the innovator’s stage to the laggard’s stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the medical device manufacturing outsourcing market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape of Medical Device Manufacturing Outsourcing Industry
Competitive Landscape & Market Insights
Companies are implementing various strategies, such as strategic alliances, medical device manufacturing outsourcing market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
Benchmark Electronics Inc. - The point of care medical device industry benefits from the company's specialized outsourcing services for medical device manufacturing. This analyst's perspective highlights the company's expertise in producing high-quality devices, ensuring regulatory compliance, and streamlining production processes for clients.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Benchmark Electronics Inc.
- Cadence Inc.
- Celestica Inc.
- Cirtec Medical
- Eurofins Scientific SE
- Flex Ltd.
- Gerresheimer AG
- Heraeus Holding GmbH
- Integer Holdings Corp.
- Jabil Inc.
- Kimball Electronics Inc.
- NN Inc.
- Nortech Systems Inc.
- Plexus Corp.
- Sanmina Corp.
- Tata Elxsi Ltd.
- TE Connectivity Ltd.
- Tecomet Inc.
- TRICOR Systems Inc.
- West Pharmaceutical Services Inc.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Medical Device Manufacturing Outsourcing Market
- In January 2024, Medtronic, a leading global healthcare solutions company, announced the expansion of its manufacturing partnership with Flextronics, a leading manufacturing solutions provider. The collaboration aimed to increase Medtronic's manufacturing capacity for cardiac and vascular products (Medtronic Press Release, 2024).
- In March 2024, Jabil Healthcare, a leading healthcare manufacturing solutions provider, acquired Mold-Tech, a specialized medical device contract manufacturer. This acquisition expanded Jabil Healthcare's capabilities in injection molding and complex assembly (Jabil Press Release, 2024).
- In April 2025, Siemens Healthineers, a German medical technology company, received FDA approval for its new Magnetom Vida MRI system, manufactured by Foxconn's subsidiary, FIH Medical. This marked the first major regulatory approval for FIH Medical's medical device manufacturing operations (Siemens Healthineers Press Release, 2025).
- In May 2025, Becton, Dickinson and Company (BD), a leading medical technology company, announced a strategic partnership with Wipro GE Healthcare, a leading provider of healthcare IT solutions and services. The partnership aimed to integrate BD's medical devices with Wipro GE Healthcare's clinical software platforms (BD Press Release, 2025).
Dive into Technavio’s robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Medical Device Manufacturing Outsourcing Market insights. See full methodology.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
246 |
|
Base year |
2024 |
|
Historic period |
2019-2023 |
|
Forecast period |
2025-2029 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 11.5% |
|
Market growth 2025-2029 |
USD 58886 million |
|
Market structure |
Fragmented |
|
YoY growth 2024-2025(%) |
10.1 |
|
Key countries |
US, China, Japan, Germany, UK, India, Canada, Brazil, France, and South Korea |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
Research Analyst Overview
- In the dynamic and intricate landscape of medical device manufacturing, outsourcing has emerged as a strategic response to the complexities of quality assurance, sterilization validation, and inventory management. This approach allows Original Equipment Manufacturers (OEMs) to focus on their core competencies while leveraging specialized expertise in areas such as change control management, computer system validation, manufacturing execution systems, design verification, material handling, sterile manufacturing, and process capability analysis. Outsourcing partners bring valuable experience in validation protocols, material traceability, regulatory compliance, and quality management systems. Cleanroom design and product lifecycle management are also critical aspects of the outsourcing relationship.
- OEMs benefit from the efficiency gains of production planning and supply chain management, as well as the production yield improvements that result from statistical process control and device testing. The outsourcing market is characterized by continuous evolution, with ongoing advancements in areas like packaging processes, contract manufacturing, and risk management. Quality control and good manufacturing practices are essential components of this landscape, ensuring that the highest standards are met throughout the manufacturing process. Moreover, the integration of manufacturing execution systems and design control enables seamless communication and collaboration between OEMs and their outsourcing partners. This results in enhanced production efficiency and improved overall quality, making medical device manufacturing outsourcing an indispensable aspect of the industry's growth and innovation.
What are the Key Data Covered in this Medical Device Manufacturing Outsourcing Market Research and Growth Report?
-
What is the expected growth of the Medical Device Manufacturing Outsourcing Market between 2025 and 2029?
-
USD 58.89 billion, at a CAGR of 11.5%
-
-
What segmentation does the market report cover?
-
The report segmented by Product (EMS, Raw materials, and Finished goods), Class Type (Class II, Class III, and Class I), Service Type (Manufacturing services, Quality assurance and testing, Device development and design services, and Regulatory and certification support), End-user (Medical device companies, Pharmaceutical and biotech firms, Hospitals and clinics, and Research institutions), and Geography (North America, Asia, Europe, and Rest of World (ROW))
-
-
Which regions are analyzed in the report?
-
North America, Asia, Europe, and Rest of World (ROW)
-
-
What are the key growth drivers and market challenges?
-
Growing focus of OEMs on reducing medical device manufacturing costs, Stringent regulatory environment
-
-
Who are the major players in the Medical Device Manufacturing Outsourcing Market?
-
Key Companies Benchmark Electronics Inc., Cadence Inc., Celestica Inc., Cirtec Medical, Eurofins Scientific SE, Flex Ltd., Gerresheimer AG, Heraeus Holding GmbH, Integer Holdings Corp., Jabil Inc., Kimball Electronics Inc., NN Inc., Nortech Systems Inc., Plexus Corp., Sanmina Corp., Tata Elxsi Ltd., TE Connectivity Ltd., Tecomet Inc., TRICOR Systems Inc., and West Pharmaceutical Services Inc.
-
Market Research Insights
- The market encompasses a diverse range of services, from total quality management and capacity planning to continuous improvement and nonconformance management. According to industry estimates, the market's value is projected to reach USD150 billion by 2025, representing a significant increase from the USD100 billion recorded in 2020. This growth can be attributed to the increasing demand for cost optimization, process improvement, and regulatory submissions. Resource allocation and audit management are crucial aspects of outsourcing, with many companies seeking to minimize downtime and ensure data integrity. Six sigma methodologies and lean manufacturing principles are increasingly being adopted to reduce defect rates and improve production scalability.
- Furthermore, the importance of supplier selection and on-time delivery cannot be overstated, with companies striving for supply chain resilience and FDA regulations compliance. Equipment maintenance, training programs, and project management are essential components of a successful outsourcing strategy. Preventive actions, design transfer, and corrective actions are also critical to maintaining high-quality standards and ensuring employee safety. Recall management and complaint handling are vital for managing risks and mitigating potential issues. ISO 13485 compliance and manufacturing automation are key trends in the market, with companies leveraging technology to enhance efficiency and reduce costs. The market's continuous evolution underscores the importance of adaptability and flexibility in today's business landscape.
We can help! Our analysts can customize this medical device manufacturing outsourcing market research report to meet your requirements.