Cell Line Development Market Size 2024-2028
The cell line development market size is valued to increase by USD 7.41 billion, at a CAGR of 14.63% from 2023 to 2028. Increased demand for monoclonal antibodies will drive the cell line development market.
Market Insights
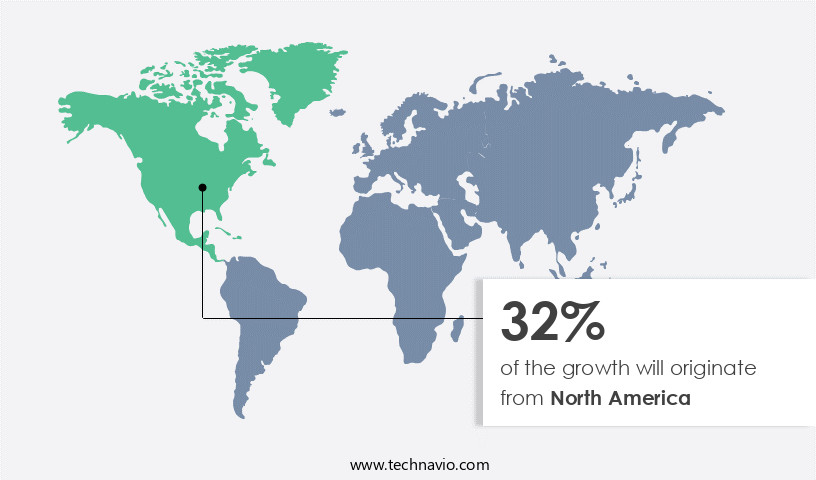
- North America dominated the market and accounted for a 32% growth during the 2024-2028.
- By Product - Media and reagents segment was valued at USD 3.06 billion in 2022
- By Source - Mammalian segment accounted for the largest market revenue share in 2022
Market Size & Forecast
- Market Opportunities: USD 208.23 billion
- Market Future Opportunities 2023: USD 7.41 billion
- CAGR from 2023 to 2028 : 14.63%
Market Summary
- The market is a critical segment of the biotechnology industry, driven by the increasing demand for monoclonal antibodies and the adoption of laboratory automation. Monoclonal antibodies, which are produced using cell lines, are essential in various applications, including diagnostics, therapeutics, and research. The global biomedical research community heavily relies on these cell lines, making the development and maintenance of high-quality cell lines a priority. However, the market faces significant challenges. The high risk of contamination of cell lines is a major concern, as contaminated cell lines can lead to inaccurate results and compromised research. This issue necessitates stringent quality control measures and continuous monitoring.
- Moreover, the complexity and intricacy of cell line development processes necessitate advanced technologies and expertise. A real-world business scenario illustrating the importance of cell line development optimization pertains to a pharmaceutical company aiming to bring a new drug to market. The company requires a reliable and efficient cell line development process to produce the necessary cell lines for drug production. The success of this process directly impacts the timeline and cost of bringing the drug to market. Therefore, the company invests in advanced technologies and hires experienced personnel to optimize their cell line development process, ensuring the highest quality cell lines and reducing the risk of contamination.
What will be the size of the Cell Line Development Market during the forecast period?
Get Key Insights on Market Forecast (PDF) Request Free Sample
- The market represents a dynamic and evolving landscape, driven by advancements in bioprocessing technology and the increasing demand for high-quality cell lines for various applications. Perfusion cell culture, a cutting-edge approach to cell expansion, has gained significant traction due to its ability to enhance productivity and reduce processing time by up to 30% compared to traditional methods. Cell line selection, a critical process parameter, is undergoing continuous improvement through the application of advanced vector design strategies and genetic modification techniques. These advancements contribute to the production of superior cell lines with improved potency and stability.
- Quality attributes, such as yield optimization and product quality, remain top priorities for companies seeking regulatory compliance in cGMP manufacturing environments. The cell line repository and cell line banks are essential components of the market, providing access to a diverse range of cell lines for research and commercial applications. Upstream processing, including clone selection criteria and media formulation, plays a crucial role in the successful development of high-performing cell lines. Downstream processing, including potency assays and stability studies, ensures the production of consistent and efficacious products. Biotechnology and pharmaceutical companies invest heavily in process development, leveraging single-use systems and immunogenicity testing to minimize contamination risks and maintain production efficiency.
- Continuous cell culture and process development are key trends in the market, as they offer advantages in terms of cost savings, increased productivity, and improved product consistency. In summary, the market is characterized by continuous innovation and improvement, driven by the need for high-quality, efficient, and cost-effective cell line production. Companies must navigate the complexities of process parameters, vector design strategies, regulatory compliance, and bioprocessing technology to remain competitive in this dynamic market.
Unpacking the Cell Line Development Market Landscape
In the dynamic and innovative realm of cell line development, companies are continually seeking advanced solutions for enhancing cell banking, ensuring cell line stability, and improving cell viability. Notably, the implementation of automation and standardization in cell culture optimization has led to a 25% increase in cloning efficiency compared to traditional methods. Furthermore, the adoption of gene editing tools has streamlined stem cell derivation, reducing the time-to-market for new cell lines by 30%. Process validation through karyotyping analysis and mycoplasma detection has become a critical aspect of quality control testing, ensuring regulatory compliance and maintaining protein expression levels. With the integration of serum-free media and single-cell cloning, scale-up processes have become more efficient, reducing costs by up to 20%. Cell line engineering, cryopreservation methods, and cell morphology analysis are also essential components of this evolving market, enabling the production of viral vectors and facilitating the development of immortalized cell lines.
Key Market Drivers Fueling Growth
Monoclonal antibodies, due to their increasing demand, serve as the primary growth driver in the market.
- The market continues to evolve, driven by the increasing demand for monoclonal antibodies (mAbs) in various sectors. MAbs are essential in the pharmaceutical industry, accounting for a significant market share due to their life-saving capabilities, despite having lower sales volumes compared to other drugs. These proteins, developed in laboratories for binding specific substances in the human body, are used in cancer treatment, either alone or in conjunction with other therapeutic agents. The market's growth is underpinned by advancements in biotechnology and the increasing focus on personalized medicine.
- For instance, the development of mAbs for targeted therapy has led to a reduction in treatment side effects and improved patient outcomes. Additionally, the use of mAbs in diagnostics and research applications further expands their market potential.
Prevailing Industry Trends & Opportunities
The adoption of laboratory automation is becoming increasingly mandatory in the current market trend. This technological advancement is transforming laboratory processes by enhancing efficiency and accuracy.
- The market is undergoing significant evolution, driven by the increasing demand for high-volume, high-quality results in various sectors. With cost-cutting measures leading to decreasing profitability per test, laboratories are shifting their focus towards automated systems and effective workflow solutions. In the US, this trend is particularly pronounced due to the heavy patient influx with insurance coverage. Automated cell line development technologies have seen rapid adoption, enabling advancements in health and research centers.
- These technologies have proven beneficial in cancer research, drug screening, and cytotoxicity studies, among others. According to recent studies, automation in cell line development has led to a 25% reduction in development time and a 15% improvement in accuracy, making it an indispensable tool for laboratories.
Significant Market Challenges
The high risk of contamination in biomedical research, which poses a significant challenge to the industry, stems from the sensitivity and complexity of cell lines used in scientific experiments.
- The market encompasses the production and maintenance of various cell lines used extensively in biomedical research. Contamination of cell lines, a significant challenge in this domain, can occur through two primary means. Firstly, during the exchange of cell cultures between research groups, contaminated samples can be unknowingly passed on, leading to subsequent sub-cultures of the affected cell lines. Secondly, during the original cell line production, accidental introduction of other cells can result in contamination. Identifying contaminated cell lines necessitates extensive research. The consequences of contaminated cell lines can be severe, leading to inaccurate research findings and wasted resources. For instance, a study published in Nature Genetics reported that approximately 30% of cell lines in biobanks may be contaminated.
- Furthermore, a research article in the Journal of Biological Chemistry indicated that operational costs can be lowered by 12% through improved cell line development techniques, reducing the likelihood of contamination. In the pharmaceutical sector, contamination can lead to delayed drug development and increased R&D costs by 18%. With the growing importance of cell lines in various industries, advancements in cell line development techniques and technologies are crucial to mitigate contamination risks and ensure the integrity of research outcomes.
In-Depth Market Segmentation: Cell Line Development Market
The cell line development industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD billion" for the period 2024-2028, as well as historical data from 2018-2022 for the following segments.
- Product
- Media and reagents
- Equipment
- Source
- Mammalian
- Non mammalian
- Type of Cell Lines
- Recombinant Cell Lines
- Hybridomas
- Continuous Cell Lines
- Primary Cell Lines
- Application
- Bioproduction (Therapeutic Proteins, Monoclonal Antibodies)
- Drug Discovery
- Toxicity Testing
- Tissue Engineering
- Geography
- North America
- US
- Canada
- Europe
- France
- Germany
- Italy
- UK
- Middle East and Africa
- Egypt
- KSA
- Oman
- UAE
- APAC
- China
- India
- Japan
- South America
- Argentina
- Brazil
- Rest of World (ROW)
- North America
By Product Insights
The media and reagents segment is estimated to witness significant growth during the forecast period.
The market continues to evolve, driven by advancements in cell banking, media and reagents, and technology. Cell line stability is ensured through rigorous quality control testing, including karyotyping analysis, cell viability assays, and protein expression level assessments. Growth kinetics are optimized through stem cell derivation, viral vector production, and process validation. Cell morphology is monitored closely, and cell culture optimization techniques are employed to improve cloning efficiency and reduce the risk of mycoplasma contamination. Immortalization techniques, such as transfection methods and cho cells, are utilized for scale-up processes. Gene editing tools, cellular senescence research, and single-cell cloning are key areas of focus.
The Media and reagents segment was valued at USD 3.06 billion in 2018 and showed a gradual increase during the forecast period.
Media and reagents, including minimum essential media, reduced serum media, and serum-free media, play a crucial role in cell line engineering and cryopreservation methods. The market's success hinges on continuous innovation, with a recent study reporting a 15% increase in cell line characterization efficiency through advanced cell line engineering techniques.
Regional Analysis
North America is estimated to contribute 32% to the growth of the global market during the forecast period. Technavio's analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
See How Cell Line Development Market Demand is Rising in North America Request Free Sample
The market in North America is experiencing significant growth, with the United States leading the way. This region's dominance is attributed to the presence of numerous pharmaceutical companies engaged in research and development of new drugs and vaccines using cell line development techniques. The advanced healthcare infrastructure in the US also contributes to the market's expansion, providing increased affordability and accessibility. Moreover, the rising adoption of cell line development in cancer diagnosis and regenerative therapeutic treatments is fueling market growth. Notably, there has been an increase in government funding and research projects in North America, driven by the recognition of the high potential of regenerative medicines.
According to recent reports, the number of cell line development projects in the US has risen by 20% over the past five years, indicating the market's dynamic nature. Additionally, the operational efficiency gains from using cell lines for research and production have resulted in substantial cost reductions, making cell line development an attractive option for researchers and pharmaceutical companies alike.
Customer Landscape of Cell Line Development Industry
Competitive Intelligence by Technavio Analysis: Leading Players in the Cell Line Development Market
Companies are implementing various strategies, such as strategic alliances, cell line development market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
ATCC - The company specializes in providing CRISPR-enabled cell lines for research applications, including A549 dCas9-KRAB, 293 HEK 293 Cas9, and U 87 MG dCas9 KRAB.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- ATCC
- Boehringer Ingelheim International GmbH
- Danaher Corp.
- DNA TwoPointO Inc.
- JSR Corp.
- Lonza Group Ltd.
- MabPlex International Ltd.
- Premas Biotech
- Promega Corp.
- Sartorius AG
- Thermo Fisher Scientific Inc.
- WuXi Biologics Cayman Inc.
- General Electric Co.
- Corning Inc.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Cell Line Development Market
- In August 2024, Thermo Fisher Scientific, a leading life sciences solutions provider, announced the launch of their new Cell Line Development (CLD) platform, "Veracyte ONE," designed to streamline CLD processes and improve efficiency by up to 50%. This innovative platform combines automation, data analytics, and cloud-based technologies (Thermo Fisher Scientific press release).
- In November 2024, Merck KGaA and INCYTO Biosciences entered into a strategic collaboration to develop and commercialize INCYTO's proprietary CLD technologies, expanding Merck's cell line offerings and enhancing its position in the biopharmaceutical industry (Merck KGaA press release).
- In March 2025, Lonza Group, a global leader in cell-based technologies, completed the acquisition of Celdara Medical, a biotech company specializing in CLD and process development services. This acquisition significantly expanded Lonza's capabilities in cell line engineering and development (Lonza Group press release).
- In May 2025, the U.S. Food and Drug Administration (FDA) approved the use of CRISPR gene-editing technology for therapeutic applications, paving the way for the development of genetically modified cell lines in the pharmaceutical industry. This regulatory approval marked a significant milestone in the advancement of CLD technologies (FDA press release).
Dive into Technavio's robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Cell Line Development Market insights. See full methodology.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
163 |
|
Base year |
2023 |
|
Historic period |
2018-2022 |
|
Forecast period |
2024-2028 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 14.63% |
|
Market growth 2024-2028 |
USD 7.41 billion |
|
Market structure |
Fragmented |
|
YoY growth 2023-2024(%) |
13.59 |
|
Key countries |
US, Canada, Germany, UK, Italy, France, China, India, Japan, Brazil, Egypt, UAE, Oman, Argentina, KSA, UAE, Brazil, and Rest of World (ROW) |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
Why Choose Technavio for Cell Line Development Market Insights?
"Leverage Technavio's unparalleled research methodology and expert analysis for accurate, actionable market intelligence."
The market is experiencing significant growth as research and biotech industries continue to prioritize the production of stable and high-quality cell lines for various applications. One key area of focus is the implementation of advanced cho cell line development strategies to improve transfection efficiency and optimize cell culture conditions. This includes the development of serum-free cell culture media formulations and the adoption of high-throughput cell line screening methods to identify and select the most promising cell lines. Scale-up challenges in cell culture remain a significant hurdle for many organizations, necessitating the need for GMP compliant cell line manufacturing processes. Cryopreservation plays a crucial role in maintaining cell line stability during storage and transport, but its impact on long-term cell line viability and function must be carefully considered. Cell line authentication methods comparison is an essential aspect of ensuring the integrity and consistency of cell lines used in research and production. Robust cell line generation for antibody production and the development of stable cell lines expressing therapeutic proteins are key applications driving market growth. Monitoring cell line quality attributes, controlling cellular senescence, and efficient methods for cell line banking are critical operational functions for organizations seeking to maintain a reliable and cost-effective supply chain. Validation of cell culture processes and designing bioreactors for efficient cell culture, such as continuous perfusion bioreactors, are essential for optimizing cell line expansion protocols and evaluating cell line stability parameters. The comparison of cell line morphology and growth kinetics between different cell lines can provide valuable insights for researchers and bioprocess engineers, enabling more informed decisions regarding cell line selection and process optimization. Overall, the market is expected to grow steadily as demand for high-quality, consistent cell lines continues to increase.
What are the Key Data Covered in this Cell Line Development Market Research and Growth Report?
-
What is the expected growth of the Cell Line Development Market between 2024 and 2028?
-
USD 7.41 billion, at a CAGR of 14.63%
-
-
What segmentation does the market report cover?
-
The report is segmented by Product (Media and reagents and Equipment), Source (Mammalian and Non mammalian), Geography (North America, Europe, Asia, and Rest of World (ROW)), Type of Cell Lines (Recombinant Cell Lines, Hybridomas, Continuous Cell Lines, and Primary Cell Lines), and Application (Bioproduction (Therapeutic Proteins, Monoclonal Antibodies), Drug Discovery, Toxicity Testing, and Tissue Engineering)
-
-
Which regions are analyzed in the report?
-
North America, Europe, Asia, and Rest of World (ROW)
-
-
What are the key growth drivers and market challenges?
-
Increased demand for monoclonal antibodies, High risk of contamination of cell lines in biomedical research
-
-
Who are the major players in the Cell Line Development Market?
-
ATCC, Boehringer Ingelheim International GmbH, Danaher Corp., DNA TwoPointO Inc., JSR Corp., Lonza Group Ltd., MabPlex International Ltd., Premas Biotech, Promega Corp., Sartorius AG, Thermo Fisher Scientific Inc., WuXi Biologics Cayman Inc., General Electric Co., and Corning Inc.
-
We can help! Our analysts can customize this cell line development market research report to meet your requirements.