North America Coronavirus Test Kits Market Size 2024-2028
The North America coronavirus test kits market size is forecast to increase by USD -1419 million, at a CAGR of -71.7% between 2023 and 2028.
- The market is experiencing significant momentum due to the integration of COVID-19 testing into routine healthcare check-ups. This trend underscores the growing importance of diagnostic testing in managing the ongoing pandemic and maintaining public health. Simultaneously, the market is witnessing the launch of new COVID-19 test kits, offering enhanced accuracy, convenience, and speed. In the fields of human genetic testing, forensic sciences, pathogen detection, oncology testing, blood screening, and POC molecular diagnostics, the demand for accurate and reliable test kits is increasing. The use of COVID-19 test kits in various applications, from infectious disease diagnosis to research and development, is expanding. However, the market faces challenges as well. The easy availability of counterfeit COVID-19 test kits poses a significant threat to market growth and consumer trust. Companies must navigate this challenge by implementing robust quality control measures and collaborating with regulatory authorities to ensure the authenticity and efficacy of their test kits.
- By focusing on innovation, quality, and regulatory compliance, market participants can capitalize on the growing demand for COVID-19 testing and effectively address the challenges presented by the counterfeit market.
What will be the size of the North America Coronavirus Test Kits Market during the forecast period?
Explore in-depth regional segment analysis with market size data - historical 2020-2022 and forecasts 2024-2028 - in the full report.
Request Free Sample
- The North American coronavirus test kits market is witnessing significant activity and trends in high-throughput screening and data reporting systems. ELISA testing, immunofluorescence assay, and lateral flow immunoassay are commonly used diagnostic techniques, undergoing clinical and external validation for assay reproducibility and calibration procedures. Genetic sequencing plays a crucial role in infection control measures and viral load quantification, while automated testing systems streamline test result interpretation. Serial dilution and standard curves are essential for performance evaluation and method comparison. Fluorescence immunoassay and chemiluminescence immunoassay contribute to quantitative results, with biosafety protocols ensuring test accuracy and safety.
- Waste disposal guidelines and infection control measures are integral to the diagnostic workflow. Cost effectiveness is a critical factor, with serial dilution, calibration procedures, and viral load quantification contributing to test affordability. Infection control measures, limit of quantitation, and assay reproducibility are key considerations for biosafety and accurate test results.
How is this market segmented?
The market research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2024-2028, as well as historical data from 2020-2022 for the following segments.
- End-user
- Government
- Non-government
- Type
- Rapid test kit
- RT-PCR
- Others
- Geography
- North America
- US
- Canada
- Mexico
- North America
By End-user Insights
The government segment is estimated to witness significant growth during the forecast period.
In response to the ongoing COVID-19 pandemic, governments at all levels have significantly increased their consumption of coronavirus test kits for public health initiatives. These programs, which include testing for both symptomatic and asymptomatic individuals, have led to a surging demand for test kits. One notable example is the White House's introduction of COVIDTests.Gov in January 2022, which enabled American households to order free at-home test kits, resulting in a substantial requirement for these kits from the federal government. Antibody tests, negative controls, and the limit of detection are essential components of these test kits, ensuring diagnostic accuracy. Assay validation and false positive rate are critical factors in the selection and implementation of these tests.
Saliva sample collection has emerged as a convenient and non-invasive alternative to nasal or throat swabs for some tests, while RT-PCR technology and reverse transcription form the basis of molecular diagnostic tests. FDA EUA authorization and CE marking certification are essential regulatory approvals for these test kits, ensuring their safety and efficacy. Home testing kits have gained popularity due to their convenience, while storage conditions and sample processing are crucial factors in maintaining test kit specificity. Point-of-care testing and rapid diagnostic tests offer quick results, making them valuable tools in controlling the spread of the virus. Quality control measures, such as internal controls and positive and negative controls, are essential in ensuring test kit sensitivity and diagnostic accuracy.
The supply chain management of test kit components, including reagents and buffers, is a critical aspect of the market's dynamics. Sample collection methods, such as oropharyngeal and nasopharyngeal swabs, are essential for accurate test results. Data analysis software plays a crucial role in interpreting test results and understanding the false negative and false positive rates. Molecular diagnostic tests, such as PCR amplification and nucleic acid extraction, are essential in identifying the presence of the virus. Antigen detection tests offer an alternative, faster testing method, while test kit sensitivity and transportation requirements are essential considerations for their implementation. In conclusion, the coronavirus test kit market is experiencing significant growth due to the ongoing pandemic and the need for widespread testing.
The market's dynamics are influenced by various factors, including test kit components, regulatory approvals, sample collection methods, and quality control measures.
The Government segment was valued at USD 3836.00 million in 2020 and showed a gradual increase during the forecast period.
Market Dynamics
Our researchers analyzed the data with 2023 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
What are the North America Coronavirus Test Kits Market drivers leading to the rise in adoption of the Industry?
- The integration of COVID-19 testing in routine healthcare check-ups is a significant market trend, as this approach enhances the efficiency and accessibility of testing services, thereby driving market growth.
- The market is experiencing significant growth due to the increasing integration of COVID-19 testing into routine healthcare check-ups. As the disease transitions from a pandemic to an endemic stage, healthcare providers are incorporating regular testing into standard medical practices. This includes screening during medical check-ups, particularly for vulnerable populations such as the elderly. The ongoing need to monitor COVID-19 alongside other respiratory illnesses, like influenza, is driving the demand for coronavirus test kits that can be used in conjunction with regular healthcare services. The integration of coronavirus testing into routine care ensures early detection and timely intervention, which is essential for managing potential outbreaks and preventing severe cases.
- Two primary types of coronavirus tests are in use: nucleic acid tests, which include reverse transcription-polymerase chain reaction (RT-PCR) technology, and antibody tests. RT-PCR technology detects the presence of the virus by reverse transcribing RNA into DNA and amplifying it for detection. Antibody tests, on the other hand, detect the presence of antibodies produced in response to the virus. Both test types undergo rigorous validation processes, including the use of negative controls and limit of detection assessments, to ensure accurate and reliable results. The FDA has granted Emergency Use Authorization (EUA) to several test kits, ensuring their safety and efficacy.
- Some tests are even approved for use with saliva sample collection, making testing more convenient and less invasive for patients. The false positive rate is a critical factor in assessing the accuracy of coronavirus test kits. Manufacturers and healthcare providers must continually monitor and address this issue to maintain confidence in the test results and prevent unnecessary interventions. In summary, the integration of coronavirus testing into routine healthcare check-ups is driving the growth of the market. The ongoing need for accurate and reliable testing, along with the convenience of saliva sample collection and FDA EUA authorization, ensures that these tests remain a crucial component of healthcare services moving forward.
What are the North America Coronavirus Test Kits Market trends shaping the Industry?
- The upcoming market trend involves the launch of new COVID-19 test kits. This significant development in the healthcare industry is a crucial response to the ongoing pandemic.
- The coronavirus test kit market in North America is witnessing significant growth due to the ongoing research for COVID-19 vaccines and treatments. Product quality and visibility are crucial factors influencing sales. Companies are utilizing various channels, including their websites and awareness programs, to promote test kit usage. Research organizations are developing multi-sample testing kits, enabling efficient testing processes. Additionally, there is a growing trend towards launching test kits with enhanced functionality, such as multiple-use kits. Kit components, including CE marking certification, are essential considerations for companies to ensure regulatory compliance.
- Effective supply chain management and sample processing are also vital for maintaining test kit specificity and accuracy. PCR amplification and serological testing are common techniques used in coronavirus testing, requiring specific storage conditions to preserve test kit effectiveness. As the market evolves, companies are prioritizing innovation and quality to meet the increasing demand for reliable and efficient test kits.
How does North America Coronavirus Test Kits Market faces challenges face during its growth?
- The proliferation of counterfeit COVID-19 test kits poses a significant challenge to the industry, threatening its growth and undermining public trust in diagnostic results.
- The market is currently confronting challenges due to the proliferation of counterfeit products. These illicit testing kits are being sold by various private clinics, labs, and intermediaries, leading to potential health risks for consumers and potential delays in medical treatment for those infected with COVID-19. On March 20, 2020, US Customs and Border Protection officials seized counterfeit coronavirus tests at O'Hare International Airport in Chicago, underscoring the severity of the issue. Nucleic acid extraction is a crucial component of molecular diagnostic tests, including those used for coronavirus detection. Internal controls are essential to ensure diagnostic accuracy and prevent false negatives.
- Data analysis software plays a vital role in processing test results efficiently and accurately. Point-of-care testing offers quicker turnaround times compared to traditional lab-based tests, making it a preferred option for many. Ensuring the authenticity and quality of coronavirus test kits is paramount to mitigate the risks associated with counterfeit products and maintain diagnostic accuracy.
Exclusive North America Coronavirus Test Kits Market Customer Landscape
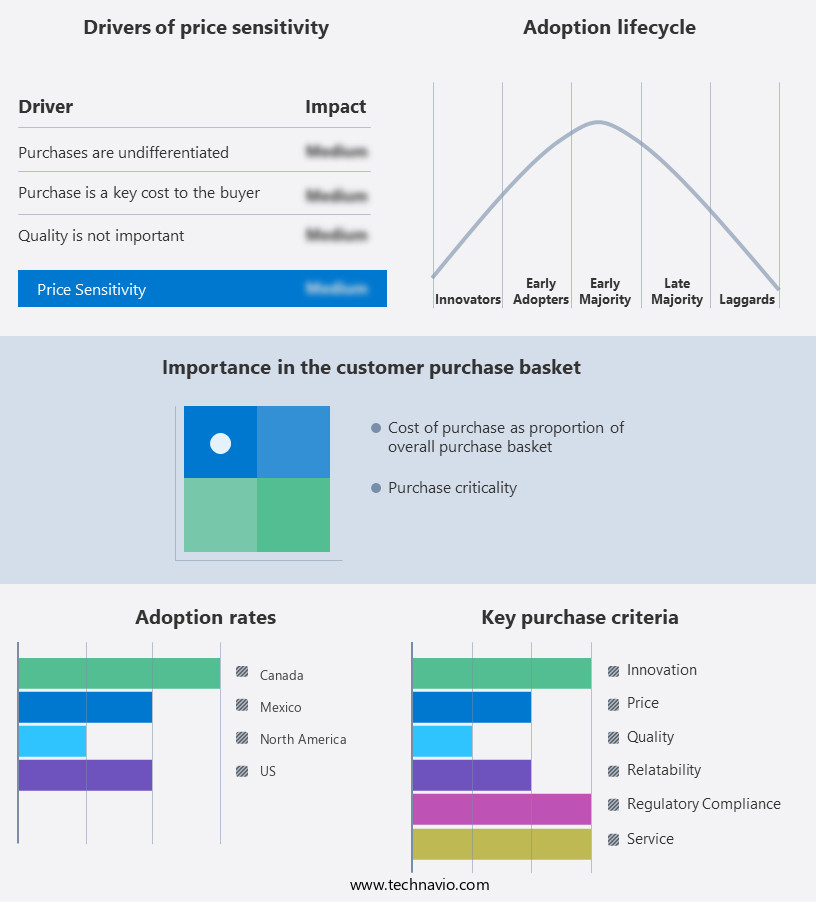
The market forecasting report includes the adoption lifecycle of the market, covering from the innovator's stage to the laggard's stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape
Key Companies & Market Insights
Companies are implementing various strategies, such as strategic alliances, market forecast partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the market.
The market research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Abbott Laboratories
- Advaite Inc.
- Becton Dickinson and Co.
- Biomedomics Inc
- bioMerieux SA
- Cellex Inc.
- Co Diagnostics Inc
- Cue Health Inc.
- Danaher Corp.
- DiaSorin SpA
- F. Hoffmann La Roche Ltd.
- Hologic Inc.
- Laboratory Corp. of America Holdings
- Mammoth Biosciences Inc.
- MyBioSource Inc.
- QIAGEN N.V.
- QuidelOrtho Corp.
- RayBiotech Life Inc.
- Robert Bosch GmbH
- Sherlock Biosciences
- Talis Biomedical Corp
- Thermo Fisher Scientific Inc.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key market players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Coronavirus Test Kits Market In North America
- In January 2024, Thermo Fisher Scientific, a leading life sciences solutions provider, announced the launch of a new real-time polymerase chain reaction (RT-PCR) test kit for detecting SARS-CoV-2, the virus causing COVID-19. The new test kit, named TaqPath COVID-19 Combo Kit, received emergency use authorization (EUA) from the U.S. Food and Drug Administration (FDA) in March 2024 (Thermo Fisher Scientific, 2024).
- In April 2024, Abbott Laboratories, a healthcare company, entered into a collaboration with the U.S. Department of Health and Human Services (HHS) to increase the production capacity of its rapid antigen test for COVID-19, BinaxNOW. The collaboration aimed to produce 50 million tests per month, with the first 15 million tests to be delivered to the federal government (Abbott, 2024).
- In February 2025, Quidel Corporation, a diagnostic company, received EUA from the FDA for its rapid antigen test, QuickVue SARS Antigen Test, to be used for point-of-care testing in various settings, including schools, workplaces, and healthcare facilities (Quidel, 2025).
- In May 2025, Roche Holding AG, a healthcare company, completed the acquisition of GenMark Diagnostics, a molecular diagnostic company. The acquisition expanded Roche's portfolio in molecular diagnostics, including the PLEX-ID RP2 System, which can detect multiple respiratory pathogens, including SARS-CoV-2 (Roche, 2025).
Research Analyst Overview
The market continues to evolve as new developments emerge in the fight against the global pandemic. Kit components, such as reagents and buffers, play a crucial role in ensuring diagnostic accuracy through techniques like reverse transcription and PCR amplification. CE marking certification and FDA EUA authorization are essential for market entry, while home testing kits offer convenience and accessibility. Serological testing, which detects antibodies, complements molecular diagnostic tests that identify the virus itself. Home testing kits require careful sample processing and storage conditions to maintain test kit specificity. Supply chain management is a critical factor, with transportation requirements and just-in-time delivery essential for maintaining a steady supply of test kits.
Negative and positive controls, internal and external, are vital for assay validation and quality control measures. Nucleic acid extraction and data analysis software are integral to molecular diagnostic tests, while saliva sample collection and oropharyngeal or nasopharyngeal swab collection methods are essential for accurate results. The market's dynamics are continually unfolding, with new testing technologies and applications emerging. For instance, antigen detection tests offer rapid results, while RT-PCR technology remains the gold standard for diagnostic accuracy. The false positive and false negative rates, along with test kit sensitivity, are essential metrics for evaluating test performance. In summary, the market is a dynamic and evolving landscape, with various sectors and applications requiring different testing methods and techniques.
The ongoing challenge is to maintain diagnostic accuracy while ensuring efficient supply chain management, rigorous quality control measures, and adherence to regulatory requirements.
Dive into Technavio's robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Coronavirus Test Kits Market in North America insights. See full methodology.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
158 |
|
Base year |
2023 |
|
Historic period |
2020-2022 |
|
Forecast period |
2024-2028 |
|
Growth momentum & CAGR |
Decelerate at a CAGR of -71.7% |
|
Market growth 2024-2028 |
USD -1419 million |
|
Market structure |
Fragmented |
|
YoY growth 2023-2024(%) |
-88.9 |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
What are the Key Data Covered in this Market Research Report?
- CAGR of the market during the forecast period
- Detailed information on factors that will drive the market growth and forecasting between 2024 and 2028
- Precise estimation of the size of the market and its contribution of the market in focus to the parent market
- Accurate predictions about upcoming market growth and trends and changes in consumer behaviour
- Growth of the market across North America
- Thorough analysis of the market's competitive landscape and detailed information about companies
- Comprehensive analysis of factors that will challenge the growth of market companies
We can help! Our analysts can customize this market research report to meet your requirements Get in touch