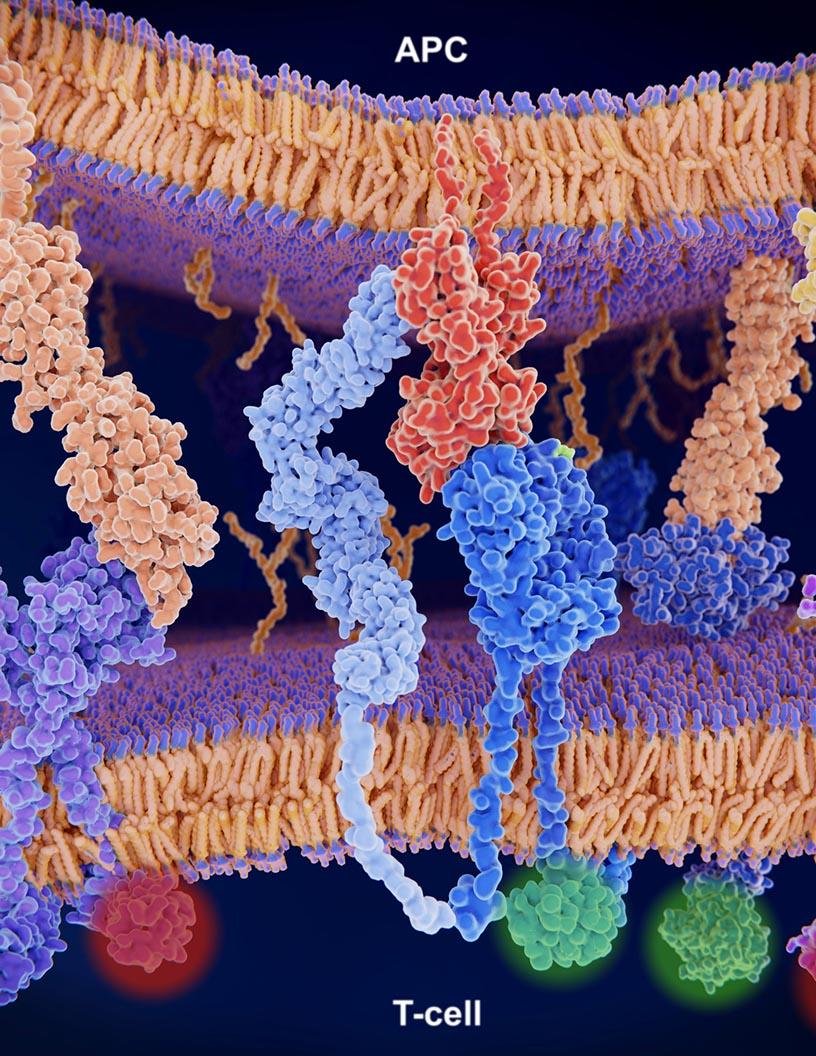
CTLA4 Inhibitors Market Size 2025-2029
The CTLA4 inhibitors market size is forecast to increase by USD 3.5 billion, at a CAGR of 19.1% between 2024 and 2029.
- The market is characterized by the high target affinity and specificity of CTLA4 inhibitors, making them an attractive therapeutic option in the field of oncology. This market's expansion is further driven by the ongoing research into new indications for CTLA4 inhibitors, broadening their potential applications and patient base. However, the lack of approved CTLA4 therapies presents a significant challenge for market growth. Despite this hurdle, companies can capitalize on the market's potential by investing in clinical trials and collaborations to bring new CTLA4 inhibitor therapies to market.
- Additionally, focusing on addressing the unique challenges associated with CTLA4 inhibitor development, such as toxicity and resistance, can help companies differentiate themselves and gain a competitive edge. Overall, the market holds immense promise, with the potential to revolutionize cancer treatment through targeted immunotherapy. Companies that effectively navigate the challenges and seize opportunities in this market will be well-positioned for success.
What will be the Size of the CTLA4 Inhibitors Market during the forecast period?
Explore in-depth regional segment analysis with market size data - historical 2019-2023 and forecasts 2025-2029 - in the full report.
Request Free Sample
The CTLA-4 inhibitors market continues to evolve, driven by advancements in Cancer Immunotherapy and the ongoing development of new treatment options. Response rates have shown significant improvement in various sectors, with ongoing clinical trials exploring the potential of CTLA-4 inhibitors in combination with other targeted therapies and immune checkpoint inhibitors. The role of drug metabolism in optimizing treatment efficacy and minimizing adverse events is a growing area of focus. Treatment guidelines are continually being updated to reflect the latest research findings, with a greater emphasis on personalized medicine and patient selection based on tumor mutational burden, microsatellite instability, and other biomarkers.
Healthcare economics and market access remain critical considerations, with pricing strategies and intellectual property rights shaping the competitive landscape. Healthcare professionals are increasingly leveraging next-generation sequencing and liquid biopsies to improve disease management and monitor treatment response. Regulatory approval and EMA approval processes are ongoing, with a focus on ensuring the safety and efficacy of new CTLA-4 inhibitors. The supply chain and distribution channels are also evolving to meet the growing demand for these therapies, with a focus on ensuring quality and reliability. Ongoing research into drug interactions and Monoclonal Antibodies is further expanding the potential applications of CTLA-4 inhibitors in precision oncology.
Phase III trials are underway to explore the long-term health outcomes of CTLA-4 inhibitor therapy, including progression-free survival and overall survival. The ongoing unfolding of market activities and evolving patterns underscores the dynamic nature of the CTLA-4 inhibitors market and its continued potential for growth and innovation.
How is this CTLA4 Inhibitors Industry segmented?
The CTLA4 inhibitors industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2025-2029, as well as historical data from 2019-2023 for the following segments.
- Type
- Monotherapy
- Combination therapy
- Distribution Channel
- Hospital pharmacies
- Retail pharmacies
- Online pharmacies
- Route Of Administration
- Intravenous
- Subcutaneous
- Geography
- North America
- US
- Canada
- Europe
- France
- Germany
- Italy
- The Netherlands
- UK
- APAC
- China
- India
- Japan
- Rest of World (ROW)
- North America
By Type Insights
The monotherapy segment is estimated to witness significant growth during the forecast period.
CTLA4 inhibitors have gained significant attention in the cancer therapeutics market due to their efficacy in treating specific subtypes of melanomas, non-small cell lung cancer (NSCLC), metastatic colorectal cancer (mCRC), and mesothelioma. These cancers are characterized by the malfunctioning of the CTLA4 protein, making them responsive to targeted therapies such as CTLA4 inhibitors, administered as monotherapy. The US Food and Drug Administration (FDA) approved YERVOY as the first monotherapy for metastatic and unresectable melanoma in 2011, increasing its popularity. Patient advocacy groups have played a crucial role in raising awareness and promoting the use of these therapies. Patent protection ensures that pharmaceutical companies can profitably invest in research and development of CTLA4 inhibitors.
Distribution channels facilitate the delivery of these therapies to healthcare professionals and patients. Phase I and II clinical trials have shown promising progression-free survival and overall survival rates, driving the market forward. FDA approval and European Medicines Agency (EMA) approval are essential regulatory milestones for market access. Pricing strategies and healthcare economics are significant factors influencing market dynamics. Personalized medicine, based on tumor mutational burden and microsatellite instability, is a promising trend. Next-generation sequencing and liquid biopsies enable accurate patient selection. Drug interactions, adverse events, and quality of life are essential considerations in treatment guidelines. Combination therapy, including CTLA4 inhibitors in conjunction with immune checkpoint inhibitors, is a growing trend.
Monoclonal antibodies and precision oncology are essential components of targeted therapy. Market access, supply chain, and regulatory approval are crucial elements of the healthcare ecosystem. Despite the advancements, generic competition and drug metabolism pose challenges to market growth. Treatment guidelines and healthcare economics continue to evolve, emphasizing the importance of ongoing clinical trials and research. The market is expected to witness significant growth due to the increasing demand for targeted therapies and the potential for combination therapy.
The Monotherapy segment was valued at USD 1.03 billion in 2019 and showed a gradual increase during the forecast period.
Regional Analysis
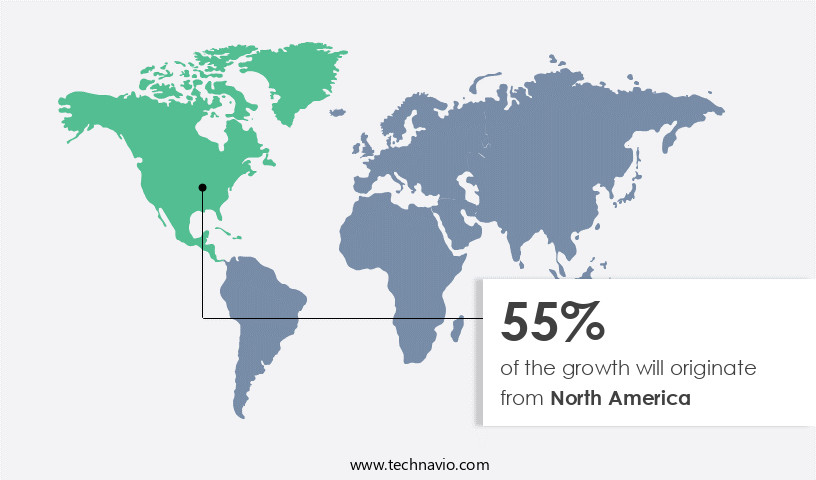
North America is estimated to contribute 55% to the growth of the global market during the forecast period.Technavio’s analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
In the US market, the growth is predominantly fueled by the sales of approved CTLA4 inhibitors, particularly YERVOY, and the rising incidence of various cancer indications, such as melanomas and NSCLC. With cancer being one of the most prevalent chronic diseases in America, the American Cancer Society anticipates that in 2024, approximately 450 new cancer cases would be reported per 100,000 people, and about 160 deaths would occur. The number of new cancer cases is projected to surge by 22% in men and 19% in women from 2020 to 2030. Melanoma, in particular, is estimated to account for approximately 100,640 new cases in 2024.
Patient advocacy groups play a crucial role in raising awareness and driving demand for effective treatments. Patent protection and distribution channels are essential factors ensuring market access to these innovative therapies. Phase I trials are underway for several CTLA4 inhibitors, showcasing ongoing research and development efforts. Progression-free survival and overall survival rates are key indicators of treatment success, driving the focus on targeted therapy. Microsatellite instability and tumor mutational burden are emerging biomarkers for patient selection in clinical trials. Pricing strategies, healthcare professionals, and regulatory approval processes are critical elements in market access and treatment guidelines. Adverse events and quality of life are essential health outcomes to consider.
Healthcare economics, personalized medicine, and disease management are key areas of focus for stakeholders. Regulatory approval, both in the US and Europe, is a significant milestone for market access and intellectual property protection. Combination therapy, drug interactions, and precision oncology are emerging trends in cancer treatment. Next-generation sequencing and liquid biopsies offer opportunities for early detection and treatment. Clinical trials, phase II and III, continue to advance our understanding of CTLA4 inhibitors' potential. Companion diagnostics and treatment guidelines play a crucial role in ensuring optimal patient outcomes. Despite market growth, generic competition from immune checkpoint inhibitors poses a challenge.
Drug metabolism and pharmacokinetics are essential considerations for drug development and distribution. Adherence to treatment guidelines and effective disease management are crucial for optimal patient outcomes. Market access, supply chain, and healthcare economics are critical factors in ensuring the successful adoption of CTLA4 inhibitors.
Market Dynamics
The CTLA4 Inhibitors Market is advancing rapidly, with Ipilimumab and Tremelimumab driving metastatic melanoma treatment and non-small cell lung cancer therapy. Combination PD-1/CTLA4 therapies and next-generation CTLA4 inhibitors enhance efficacy, while tumor-activated antibodies improve precision. The CTLA4 inhibitors market and oncology immunotherapy market fuel growth, with monotherapy CTLA4 market gaining momentum. Low-toxicity CTLA4 therapies for cancer and AI-driven drug discovery for CTLA4 inhibitors address patient needs. Ipilimumab for metastatic melanoma treatment, Tremelimumab for non-small cell lung cancer, next-generation CTLA4 inhibitors for cancer, combination PD-1/CTLA4 therapies 2025, tumor-specific CTLA4 antibodies for immunotherapy, personalized CTLA4 immunotherapy for cancer patients, CTLA4 inhibitors for advanced cancers, and cost-effective CTLA4 treatments for oncology shape the industry’s future.
Our researchers analyzed the data with 2024 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
What are the key market drivers leading to the rise in the adoption of CTLA4 Inhibitors Industry?
- The high target affinity and specificity of CTLA-4 inhibitors are the primary factors driving the market's growth, making them an essential class of therapeutics in cancer immunotherapy.
- The advanced or recurring cancer treatment landscape faces challenges in terms of drug responsiveness and patient compliance due to the severe side effects of existing therapies like chemotherapy, surgery, and radiation. CTLA4 inhibitors, a type of immune checkpoint inhibitor that targets CTLA4 (a protein on T-cells), have emerged as a promising solution. These therapies have shown potential in addressing unmet needs by enhancing the immune system's ability to fight cancer cells. Clinical trials are underway to assess the efficacy and safety of CTLA4 inhibitors in various cancer types. Next-generation sequencing and tumor mutational burden are essential tools in patient selection for these trials.
- Healthcare professionals closely monitor health outcomes to ensure optimal treatment responses and minimize side effects. Pricing strategies for CTLA4 inhibitors are a significant consideration, as they can be costly. The entrance of generic competition may influence pricing dynamics and expand patient access to these therapies. Immune checkpoint inhibitors, including CTLA4 inhibitors, have demonstrated success in phase II trials, further emphasizing their potential in cancer treatment. In conclusion, the development and implementation of CTLA4 inhibitors represent a significant step forward in addressing the limitations of current cancer treatments. Ongoing clinical trials, patient selection strategies, and pricing considerations are crucial factors shaping the future of this therapeutic class.
What are the market trends shaping the CTLA4 Inhibitors Industry?
- CTLA-4 research indications are gaining significant attention in the medical community due to their potential expansion in various applications. The emerging market trend reflects the growing importance of CTLA-4 in disease treatment and prevention.
- CTLA-4 inhibitors, specifically YERVOY and OPDIVO in combination, have shown promising results in cancer immunotherapy, particularly in the treatment of metastatic melanoma and NSCLC. These drugs have the potential to expand their application to various other oncology indications through ongoing clinical trials. BMS is currently investigating the use of OPDIVO in combination with YERVOY and other anti-cancer agents for several types of tumors, including lung, head and neck, liver, kidney, bladder, and stomach. This strategy aligns with the company's research and development focus on enhancing the marketed products, specifically OPDIVO and YERVOY, for both first- and second-line therapy in new indications.
- The response rate and quality of life improvements, along with regulatory approvals from bodies such as the EMA, make these treatments valuable additions to healthcare economics and personalized medicine. However, it is crucial to monitor and manage adverse events associated with these therapies to ensure safe and effective treatment.
What challenges does the CTLA4 Inhibitors Industry face during its growth?
- The absence of approved CTLA-4 therapies poses a significant challenge to the industry's growth trajectory. CTLA-4 is a critical immune checkpoint protein, and the development of effective therapies targeting this molecule holds immense potential for advancing cancer treatment. However, despite significant research efforts, no approved CTLA-4 therapies are currently available in the market, hindering industry progress.
- The market faces significant challenges due to the limited number of approved drugs for cancer treatment. CTLA4 inhibitors, such as YERVOY, exhibit potent anticancer effects, but their use as monotherapy is hindered by drug toxicity. Furthermore, therapeutic resistance is a major hurdle in managing advanced cancers. Consequently, investment in CTLA4 inhibitor development is relatively low, with only one approved drug in the market. YERVOY, which is approved for metastatic or unresectable melanoma, is also used in combination with other therapies for treating various cancers, including NSCLC, RCC, and mCRC. In the realm of precision oncology, the integration of CTLA4 inhibitors with other treatments, such as monoclonal antibodies and targeted therapies, is a promising approach to enhance treatment efficacy and mitigate toxicity.
- Furthermore, the application of liquid biopsies in disease management can aid in the early detection of therapeutic resistance and facilitate timely intervention. Phase III trials are ongoing to explore the potential of CTLA4 inhibitors in combination therapy and to develop companion diagnostics to optimize treatment selection and drug interactions.
Exclusive Customer Landscape
The ctla4 inhibitors market forecasting report includes the adoption lifecycle of the market, covering from the innovator’s stage to the laggard’s stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the ctla4 inhibitors market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape
Key Companies & Market Insights
Companies are implementing various strategies, such as strategic alliances, ctla4 inhibitors market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
Agenus Inc. - The company specializes in the development of CTLA-4 inhibitors, including MK-4830, UGN-301, and AGEN2373, which are potential game-changers in cancer immunotherapy research. These inhibitors aim to enhance the body's immune response against cancer cells, offering significant therapeutic benefits.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Agenus Inc.
- Akeso Inc.
- AstraZeneca Plc
- Bio Techne Corp.
- BioNTech SE
- Bristol Myers Squibb Co.
- HBM Holdings Ltd.
- Innovent Biologics Inc.
- Jiangsu Hengrui Pharmaceuticals Co. Ltd.
- Merck and Co. Inc.
- Ono Pharmaceutical Co. Ltd.
- Shanghai Fosun Pharmaceutical Group Co. Ltd.
- Xencor Inc.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in CTLA4 Inhibitors Market
- In January 2024, Merck KGaA and Incyte Corporation announced the US Food and Drug Administration (FDA) approval of their CTLA-4 inhibitor, Opdivo (nivolumab) in combination with Yervoy (ipilimumab) for the first-line treatment of adults with unresectable or metastatic microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) colorectal cancer (CRC). (Merck KGaA Press Release, 2024)
- In March 2024, Bristol Myers Squibb (BMS) and Exact Sciences announced a strategic collaboration to explore the potential of combining BMS' CTLA-4 inhibitor, Opdivo, with Exact Sciences' colorectal cancer screening test, Cologuard. The partnership aimed to identify and treat patients with early-stage colorectal cancer using a combination of precision oncology and screening. (Bristol Myers Squibb Press Release, 2024)
- In May 2024, AstraZeneca and Merck & Co. Inc. (MSD) announced the initiation of a global Phase 3 trial (IMblaze370) to evaluate the efficacy and safety of their CTLA-4 inhibitor, Imfinzi (durvalumab), in combination with tremelimumab, an anti-TIM-3 monoclonal antibody, for the treatment of advanced non-small cell lung cancer (NSCLC). (AstraZeneca Press Release, 2024)
- In April 2025, Pfizer and Merck KGaA's biopharmaceutical division, Pfizer and Merck KGaA, announced the FDA approval of their CTLA-4 inhibitor, Bavencio (avelumab), in combination with axitinib, for the first-line treatment of advanced renal cell carcinoma (RCC). The approval marked the first FDA approval for a combination of two immunotherapies for the first-line treatment of RCC. (Pfizer Press Release, 2025)
Research Analyst Overview
- The CTLA-4 inhibitor market is experiencing significant activity and trends in the realm of molecular diagnostics and immune modulation. Preclinical studies are underway to explore synthetic lethality and resistance mechanisms, while Medical Devices and antibody-drug conjugates are being developed to enhance treatment adherence and safety profile. Cytokine release syndrome and hematological toxicity are key concerns, necessitating rigorous health technology assessment and risk-benefit assessment. Disease burden and mortality rate remain high, driving the need for innovative therapeutic indexes and clinical practice guidelines. Post-market surveillance and educational materials are essential for ensuring value-based healthcare and patient support programs. Infusion reactions and tumor microenvironment are also areas of focus in the ongoing drug development of CTLA-4 inhibitors.
- Immuno-oncology approaches, including car T-cell therapy and oncolytic viruses, are gaining traction in the field. Clinical development is progressing, with Diagnostic Imaging playing a crucial role in monitoring treatment efficacy and assessing therapeutic response. The safety profile of CTLA-4 inhibitors is a critical consideration, with ongoing efforts to minimize infusion reactions and optimize therapeutic indexes. The market is dynamic, with ongoing research and innovation shaping the future of CTLA-4 inhibitor therapeutics.
Dive into Technavio’s robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled CTLA4 Inhibitors Market insights. See full methodology.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
204 |
|
Base year |
2024 |
|
Historic period |
2019-2023 |
|
Forecast period |
2025-2029 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 19.1% |
|
Market growth 2025-2029 |
USD 3500.2 million |
|
Market structure |
Concentrated |
|
YoY growth 2024-2025(%) |
15.6 |
|
Key countries |
US, Canada, Germany, UK, France, China, Japan, Italy, India, and The Netherlands |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
What are the Key Data Covered in this CTLA4 Inhibitors Market Research and Growth Report?
- CAGR of the CTLA4 Inhibitors industry during the forecast period
- Detailed information on factors that will drive the growth and forecasting between 2025 and 2029
- Precise estimation of the size of the market and its contribution of the industry in focus to the parent market
- Accurate predictions about upcoming growth and trends and changes in consumer behaviour
- Growth of the market across North America, Europe, Asia, and Rest of World (ROW)
- Thorough analysis of the market’s competitive landscape and detailed information about companies
- Comprehensive analysis of factors that will challenge the CTLA4 inhibitors market growth of industry companies
We can help! Our analysts can customize this CTLA4 inhibitors market research report to meet your requirements.