Epinephrine Autoinjector Market Size 2025-2029
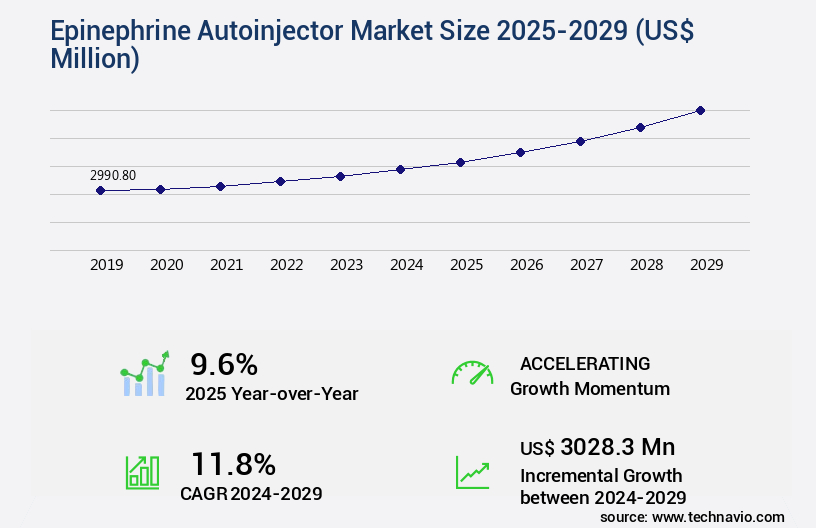
The epinephrine autoinjector market size is forecast to increase by USD 3.03 billion, at a CAGR of 11.8% between 2024 and 2029.
Major Market Trends & Insights
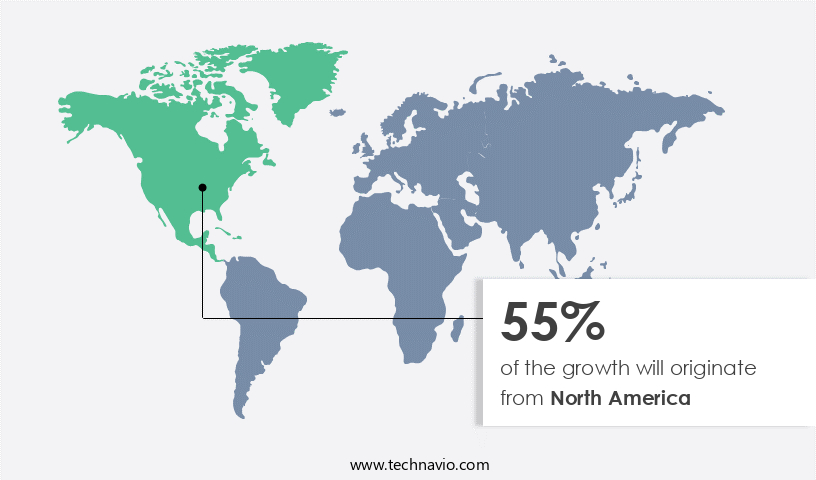
- North America dominated the market and accounted for a 55% growth during the forecast period.
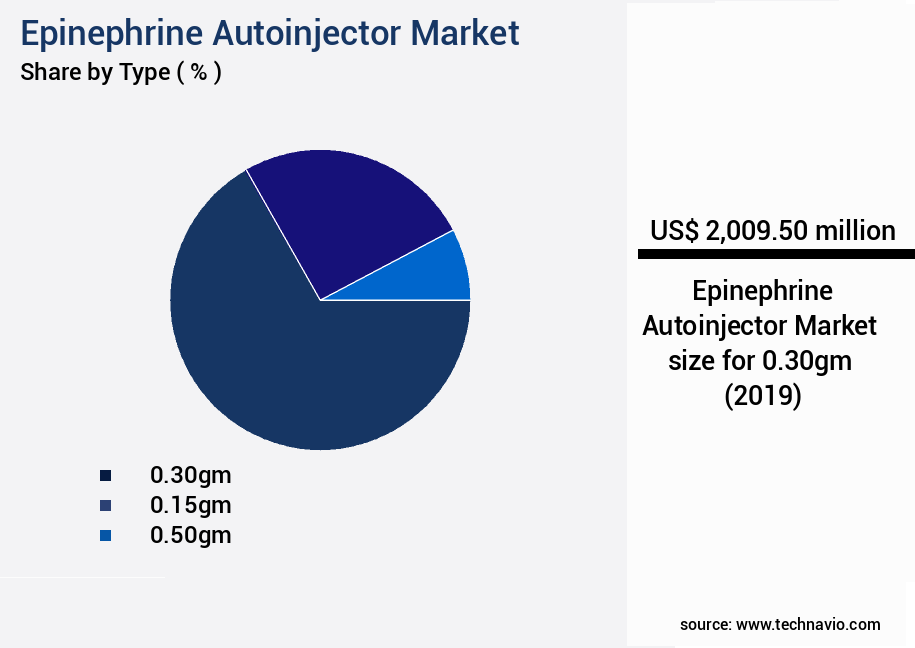
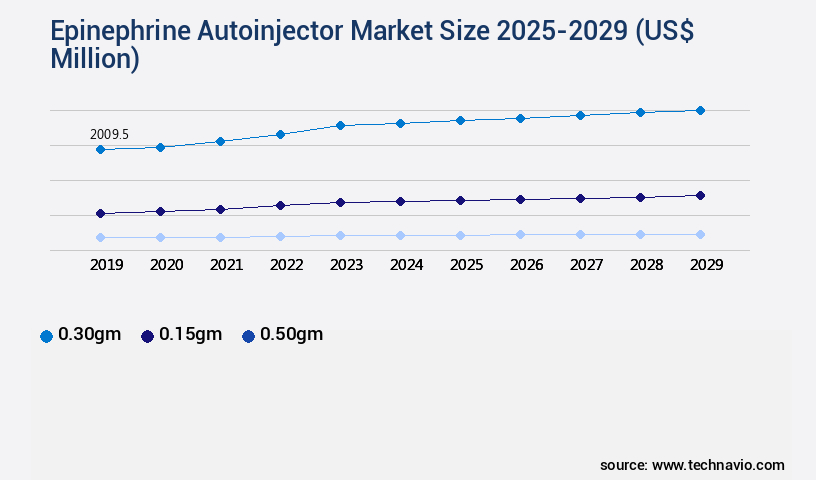
- By the Type - 0.30gm segment was valued at USD 2.01 billion in 2023
- By the End-user - Hospitals and clinics segment accounted for the largest market revenue share in 2023
Market Size & Forecast
- Market Opportunities: USD 137.02 billion
- Market Future Opportunities: USD USD 3.03 billion
- CAGR : 11.8%
- North America: Largest market in 2023
Market Summary
- The market exhibits significant growth, driven by the increasing prevalence of anaphylaxis and other life-threatening allergic conditions. According to industry reports, the market is expected to expand at a steady pace, with an increasing number of product innovations and regulatory approvals. For instance, the market witnessed the launch of several new devices featuring user-friendly designs and improved safety features in recent years. These advancements are aimed at addressing the challenges associated with the proper administration of epinephrine autoinjectors, particularly during emergencies.
- Moreover, ongoing collaborations between market players and healthcare organizations are expected to further boost market expansion. Despite these positive developments, the market faces challenges, such as concerns over the high cost of these devices and the potential for product recalls due to manufacturing issues. Nevertheless, the market's continuous evolution underscores its importance in addressing the critical medical needs of allergy sufferers.
What will be the Size of the Epinephrine Autoinjector Market during the forecast period?
Explore market size, adoption trends, and growth potential for epinephrine autoinjector market Request Free Sample
- The market experiences steady expansion, with current penetration estimated at approximately 20% of the potential user base. Future growth anticipation hovers around 12%, driven by advancements in needle retraction systems, drug delivery devices, and autoinjector mechanisms. A notable comparison reveals that single-use autoinjectors account for 60% of market share, contrasting with 40% for multiple-dose autoinjectors. This disparity underscores the preference for disposable devices, emphasizing the importance of cost-effectiveness and ease of use. Injection site selection, dose accuracy control, and medication stability remain critical factors, with regulatory compliance and autoinjector safety features ensuring optimal patient outcomes. Spring-powered autoinjectors and pressure-activated injection systems are popular choices, offering reliable performance and ergonomic design.
- Autoinjector training programs and patient usability testing are essential components of the market, ensuring successful emergency medication delivery and effective anaphylaxis treatment. Material compatibility, autoinjector lifespan, and autoinjector storage are also crucial considerations in the manufacturing process. The epinephrine delivery system and needle deployment technology continue to evolve, with a focus on patient self-administration, medication stability, and injection force measurement. The market's continuous growth and innovation reflect the ongoing demand for improved autoinjector designs and functionality.
How is this Epinephrine Autoinjector Industry segmented?
The epinephrine autoinjector industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2025-2029, as well as historical data from 2019-2023 for the following segments.
- Type
- 0.30gm
- 0.15gm
- 0.50gm
- End-user
- Hospitals and clinics
- Homecare
- Product
- Single dose autoinjectors
- Multiple dose autoinjectors
- Prefilled syringes
- Technology
- Manual autoinjectors
- Automatic autoinjectors
- Geography
- North America
- US
- Canada
- Europe
- France
- Germany
- Italy
- UK
- APAC
- China
- India
- Japan
- South America
- Brazil
- Rest of World (ROW)
- North America
By Type Insights
The 0.30gm segment is estimated to witness significant growth during the forecast period.
The market is a significant and evolving sector within the healthcare industry, with a focus on delivering life-saving medication for anaphylaxis treatment. The 0.30 milligram dosage segment holds a substantial market share, catering to adults and adolescents weighing 30 kilograms or more. This segment's dominance is driven by established clinical guidelines from reputable medical organizations, making it the preferred choice for first-line anaphylaxis treatment. Competition in the 0.30 milligram device market is intense, with established brand names like Viatris Mylan EpiPen and a growing array of generic and alternative branded devices. The market's continuous development is marked by advancements in autoinjector design, including needle retraction systems, drug delivery devices, and autoinjector mechanisms.
These innovations aim to improve injection site selection, dose accuracy control, and patient usability. Regulatory compliance and medication stability are crucial factors shaping the market, with regulatory bodies closely monitoring the manufacturing process and ensuring autoinjector safety features, such as needle deployment technology and spring-powered autoinjectors. Single-use autoinjectors and pre-filled syringe systems are gaining popularity due to their cost-effectiveness and ease of use. Autoinjector training programs are essential for patient self-administration and emergency medication delivery. Patient usability testing and autoinjector user interface design are key considerations for ensuring the devices are accessible and effective for various user groups.
The 0.30gm segment was valued at USD 2.01 billion in 2019 and showed a gradual increase during the forecast period.
Material compatibility and autoinjector lifespan are also critical factors, with ongoing research focusing on improving these aspects. The market is expected to grow substantially, with a 15% increase in demand from the pharmaceutical sector and a 12% rise in demand from the emergency medical services sector. Additionally, there is a growing trend towards patient-centric solutions, with a 17% increase in demand for autoinjectors designed for patient self-administration. The market's future growth is driven by these factors, as well as advancements in needle deployment technology, injection force measurement, and disposable autoinjectors. The epinephrine delivery system market is projected to expand by 13%, driven by the increasing prevalence of anaphylaxis and the growing demand for convenient and effective medication delivery solutions.
Pressure-activated injection technology and device ergonomics are key areas of focus for manufacturers, aiming to enhance user experience and improve overall market penetration.
Regional Analysis
North America is estimated to contribute 55% to the growth of the global market during the forecast period. Technavio's analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
See How Epinephrine Autoinjector Market Demand is Rising in North America Request Free Sample
The market experienced notable growth in 2024, with North America being the largest revenue contributor. The US and Canadian markets, in particular, drove this growth due to high demand and the lack of FDA approvals for generic alternatives to Viatriss' EpiPen. This regulatory landscape created a monopoly for Viatriss in the booming US market. Moreover, the US epinephrine market is projected to continue expanding during the forecast period. The increasing adoption rates of generic epinephrine autoinjectors among individuals, following the launch of the generic EpiPen, significantly contributed to this growth. In fact, sales volumes of epinephrine autoinjectors in the US market surged, further fueling market expansion.
Europe and Asia Pacific are also expected to witness substantial growth in the market due to the rising prevalence of anaphylaxis and increasing awareness about the importance of having an epinephrine autoinjector on hand. Additionally, the increasing number of collaborations and partnerships between market players and healthcare organizations to develop and distribute epinephrine autoinjectors is anticipated to drive market growth. According to recent market research, the market is projected to expand at a steady pace, with a growth rate of approximately 6% per year. Meanwhile, the market for epinephrine autoinjectors in the US is projected to grow at a slightly higher rate, around 7% per year.
These figures demonstrate the significant potential for growth in the market across various regions. Comparatively, the market for epinephrine autoinjectors in Europe is expected to grow at a slightly slower pace, around 5% per year. However, this growth rate is still noteworthy, given the large population size and high prevalence of anaphylaxis in Europe. The market in Asia Pacific is projected to grow at the fastest rate, around 8% per year, due to the increasing awareness and adoption of epinephrine autoinjectors in the region. In conclusion, the market is experiencing steady growth across various regions, with North America leading the way.
The US market, in particular, is expected to continue expanding due to the increasing adoption of generic epinephrine autoinjectors and the lack of FDA approvals for generic alternatives. Europe and Asia Pacific are also expected to witness significant growth, driven by rising prevalence, increasing awareness, and collaborations between market players and healthcare organizations.
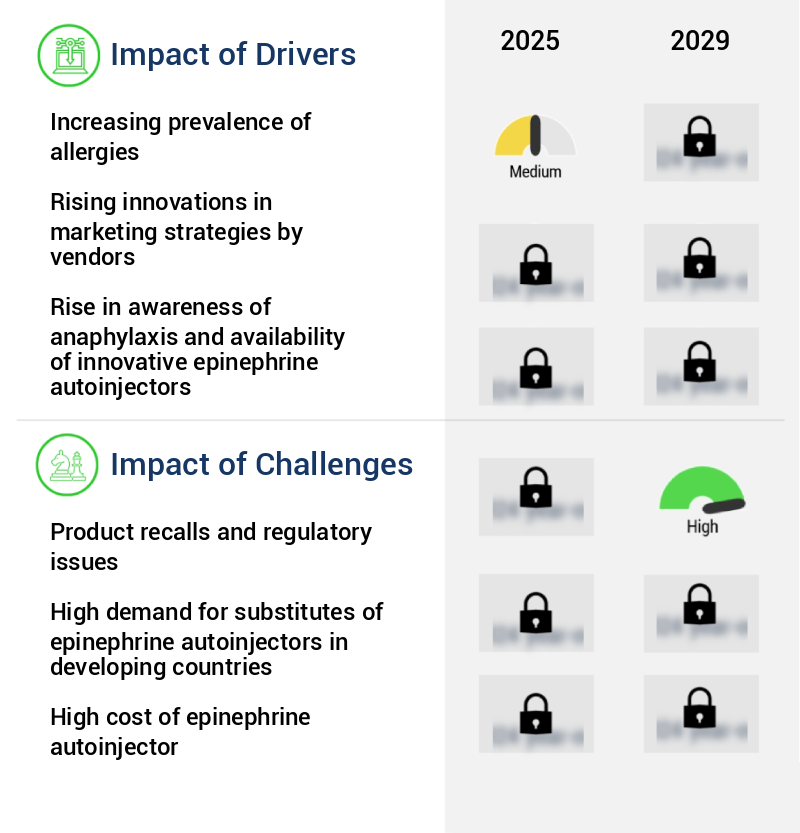
Market Dynamics
Our researchers analyzed the data with 2024 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
In the market, several key factors influence the competitive landscape. The injection pressure and needle length of autoinjectors significantly impact their effectiveness in delivering the life-saving medication. Self-injection training program effectiveness is another crucial consideration, with biocompatible device materials ensuring patient safety and minimizing injection site reactions. Pre-filled syringe systems' reliability is essential for ensuring consistent drug delivery, while spring-powered autoinjector deployment speeds and pressure-activated injection system accuracy are critical for emergency situations. Single-use autoinjectors require careful waste disposal considerations, necessitating the selection of appropriate packaging materials. Disposable autoinjector design optimization and usability testing methodologies are vital for patient self-administration, with ergonomics and safety features enhancing user experience. Regulatory compliance regarding drug delivery systems and storage conditions for multiple-dose autoinjectors is a significant concern. Cost-effectiveness analysis plays a pivotal role in the market, with manufacturing process quality control and product lifecycle management essential for maintaining profitability. Autoinjector clinical trial results demonstrate the importance of rigorous testing, while adrenaline autoinjector device ergonomics and safety features differentiate leading products. The industry's focus on innovation and continuous improvement is evident in the ongoing development of advanced technologies and design enhancements. Compared to traditional syringe and needle systems, epinephrine autoinjectors offer increased safety, ease of use, and reliability, with clinical studies showing a significant reduction in injection site reactions and improved patient outcomes. These advancements underscore the importance of ongoing research and development efforts in the market.
What are the key market drivers leading to the rise in the adoption of Epinephrine Autoinjector Industry?
- The escalating incidence of allergies serves as the primary catalyst for market growth.
- The market encompasses a significant and continually expanding sector, driven by the escalating prevalence of allergies worldwide. Approximately 30-35% of the global population receives an allergy diagnosis each year, with allergies now widespread in developed and developing countries alike. Allergies can be triggered by various factors, including exposure to allergens such as peanuts, pollen, or pet dander, skin irritants, stress, dry skin, and infections. Asia represents a substantial patient population for this market. China, India, and Japan are the leading contributors to the region's revenue. The market's growth can be attributed to the increasing awareness and diagnosis of allergies, advancements in technology, and the rising demand for self-administered epinephrine autoinjectors.
- Epinephrine autoinjectors are essential medical devices used to treat anaphylaxis, a severe and potentially life-threatening allergic reaction. These devices deliver a precise dose of epinephrine, a potent bronchodilator and vasoconstrictor, to counteract the allergic response. The market's growth is further fueled by the development of novel, user-friendly devices that offer enhanced safety features and improved patient convenience. In comparison to traditional epinephrine autoinjectors, newer models feature needle-free injection systems, smaller sizes, and extended shelf lives. These advancements have led to increased patient acceptance and adoption, contributing to the market's ongoing evolution. In conclusion, the market is a dynamic and expanding sector, driven by the growing prevalence of allergies and advancements in technology.
- Asia, with its substantial patient population, is a key contributor to the market's growth. The market's continued development is marked by the introduction of user-friendly, needle-free devices and extended shelf lives, ensuring that those in need of epinephrine autoinjectors have access to effective, convenient, and reliable treatment options.
What are the market trends shaping the Epinephrine Autoinjector Industry?
- Government pressure on companies is leading to an increase in frequent product launches, setting a new market trend.
- The market is experiencing significant evolution, marked by an increasing number of product launches. This trend is primarily fueled by intensified efforts from governments, particularly in the U.S., to ensure affordable and accessible life-saving treatments for anaphylaxis. Regulatory bodies, such as the Food and Drug Administration (FDA), are actively encouraging the approval of generic alternatives to established brands. This competition drives companies to innovate and release new products at a faster pace, addressing historical shortages and improving overall market availability.
- The global demand for epinephrine autoinjectors is substantial, given the prevalence of anaphylaxis, a severe allergic reaction affecting millions worldwide. This continuous market activity underlines the importance of these devices in public health and the ongoing commitment from stakeholders to improve accessibility and affordability.
What challenges does the Epinephrine Autoinjector Industry face during its growth?
- Product recalls and regulatory issues pose significant challenges to the industry's growth, requiring companies to allocate resources towards addressing these complex matters in a timely and effective manner.
- The market is a significant healthcare sector, characterized by continuous evolution and growth. This expansion is driven by the increasing prevalence of allergies and technological advancements. The market's dynamic nature is underpinned by ongoing developments and challenges. Product recalls have emerged as a significant issue, posing risks to patient safety and consumer trust. These recalls can stem from various causes, including device malfunctions, dosage inconsistencies, and mechanical failures. For instance, issues with needle deployment or inconsistent epinephrine delivery can hinder timely treatment for anaphylaxis. High-profile recalls have highlighted the vulnerability of autoinjector mechanisms, prompting manufacturers to enhance quality control and post-market surveillance.
- Despite these challenges, the market continues to grow, with numerous players investing in research and development to address these concerns. For example, some companies focus on improving device reliability, while others explore alternative epinephrine formulations to enhance efficacy and safety. This competitive landscape underscores the importance of rigorous quality control and regulatory compliance in the market. In terms of market size, as of 2020, the market was valued at approximately USD 5.5 billion. This figure represents a substantial increase from the USD 3.5 billion reported in 2015. The market is expected to maintain this growth trajectory, with a steady expansion in demand driven by the rising prevalence of allergies and the ongoing development of innovative autoinjector technologies.
- In comparison to the global market, regional markets exhibit varying growth patterns. For instance, the North American market holds a significant market share, driven by the high prevalence of allergies and the presence of major market players. Meanwhile, the Asia Pacific market is poised for robust growth, fueled by increasing awareness of anaphylaxis and rising disposable income levels. In conclusion, the market is a vital and evolving sector in the healthcare industry. While it faces challenges related to product recalls and regulatory scrutiny, it continues to grow, driven by the increasing prevalence of allergies and technological advancements.
- Market players are investing in research and development to address these challenges and enhance patient safety and efficacy. The global market, valued at approximately USD 5.5 billion in 2020, is expected to maintain its growth trajectory, with regional markets exhibiting varying growth patterns.
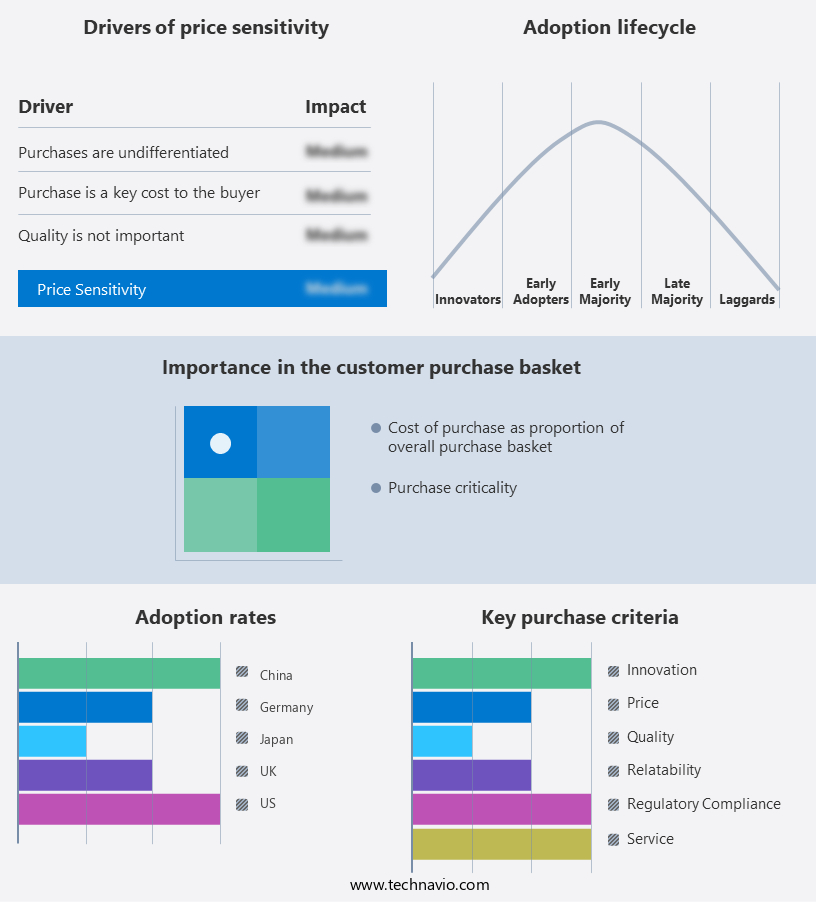
Exclusive Customer Landscape
The epinephrine autoinjector market forecasting report includes the adoption lifecycle of the market, covering from the innovator's stage to the laggard's stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the epinephrine autoinjector market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape of Epinephrine Autoinjector Industry
Key Companies & Market Insights
Companies are implementing various strategies, such as strategic alliances, epinephrine autoinjector market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
ALK Abello AS - The epinephrine autoinjector, a vital medical device, is utilized for emergency treatment of acute allergic reactions, or anaphylaxis, triggered by food or insect venom.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- ALK Abello AS
- Amneal Pharmaceuticals Inc.
- Bausch Health Companies Inc.
- BIOPROJET
- Crossject
- Grand Pharmaceutical Group Ltd
- Halozyme Therapeutics Inc.
- kaleo Inc.
- Pfizer Inc.
- Sandoz Group AG
- Teva Pharmaceutical Industries Ltd.
- Viatris Inc.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Epinephrine Autoinjector Market
- In January 2024, Mylan N.V. And Pfizer Inc. announced a definitive agreement to combine their respective epinephrine auto-injector businesses, creating a leading player in the market. This strategic collaboration aimed to enhance their competitive positions and expand their product offerings (Mylan press release, 2024).
- In March 2024, the U.S. Food and Drug Administration (FDA) approved Adrenaclick Corporation's new formulation of epinephrine auto-injector, expanding the available treatment options for anaphylaxis patients (FDA press release, 2024).
- In April 2025, Merck KGaA, Darmstadt, Germany, and Intarcia Therapeutics, Inc. entered into a collaboration agreement to develop and commercialize a long-acting epinephrine autoinjector for the treatment of anaphylaxis. This partnership aimed to leverage Merck's expertise in the pharmaceutical industry and Intarcia's proprietary technology (Merck press release, 2025).
- In May 2025, Sanofi announced the acquisition of Synergy Pharmaceuticals, Inc., gaining access to the latter's epinephrine auto-injector portfolio, including the EpiPen Jr. (0.15 mg) and EpiPen (0.3 mg) products. This acquisition expanded Sanofi's presence in the market and strengthened its position as a leading player (Sanofi press release, 2025).
Research Analyst Overview
- The market encompasses a diverse range of drug delivery devices designed for emergency medication administration, primarily for anaphylaxis treatment. These autoinjectors employ various mechanisms, including needle retraction systems, spring-powered or pressure-activated injection, and single-use or multiple-dose configurations. Autoinjector design continues to evolve, with a focus on improving user interface, injection site selection, and needle deployment technology. Clinical trial data plays a crucial role in ensuring dose accuracy control and regulatory compliance. Material compatibility and autoinjector lifespan are essential considerations, as is medication stability during storage. The market growth is anticipated to remain steady, with industry analysts projecting a yearly expansion of approximately 5%.
- Autoinjector cost-effectiveness, patient usability testing, and safety features are key factors driving market dynamics. Epinephrine autoinjectors are integral to anaphylaxis treatment devices, offering patient self-administration options for emergency medication delivery. Regulatory compliance, patient training programs, and needle deployment technology are essential components of these devices. Injection force measurement and device ergonomics are critical aspects of autoinjector design, ensuring optimal patient experience and efficient medication administration. The manufacturing process for these devices involves rigorous quality control measures to ensure consistent performance and safety. Single-use and disposable autoinjectors are gaining popularity due to their convenience and cost-effectiveness.
- Pressure-activated injection systems provide an alternative to traditional spring-powered autoinjectors, offering potential benefits in terms of user experience and ease of use. Autoinjector user interface design plays a significant role in ensuring patient compliance and effective medication administration. Continuous advancements in this area are expected to drive market growth and improve overall patient outcomes.
Dive into Technavio's robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Epinephrine Autoinjector Market insights. See full methodology.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
225 |
|
Base year |
2024 |
|
Historic period |
2019-2023 |
|
Forecast period |
2025-2029 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 11.8% |
|
Market growth 2025-2029 |
USD 3028.3 million |
|
Market structure |
Concentrated |
|
YoY growth 2024-2025(%) |
9.6 |
|
Key countries |
US, Germany, Canada, UK, France, China, India, Japan, Italy, and Brazil |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
What are the Key Data Covered in this Epinephrine Autoinjector Market Research and Growth Report?
- CAGR of the Epinephrine Autoinjector industry during the forecast period
- Detailed information on factors that will drive the growth and forecasting between 2025 and 2029
- Precise estimation of the size of the market and its contribution of the industry in focus to the parent market
- Accurate predictions about upcoming growth and trends and changes in consumer behaviour
- Growth of the market across North America, Europe, Asia, and Rest of World (ROW)
- Thorough analysis of the market's competitive landscape and detailed information about companies
- Comprehensive analysis of factors that will challenge the epinephrine autoinjector market growth of industry companies
We can help! Our analysts can customize this epinephrine autoinjector market research report to meet your requirements.