Mucus Clearance Devices Market Size 2026-2030
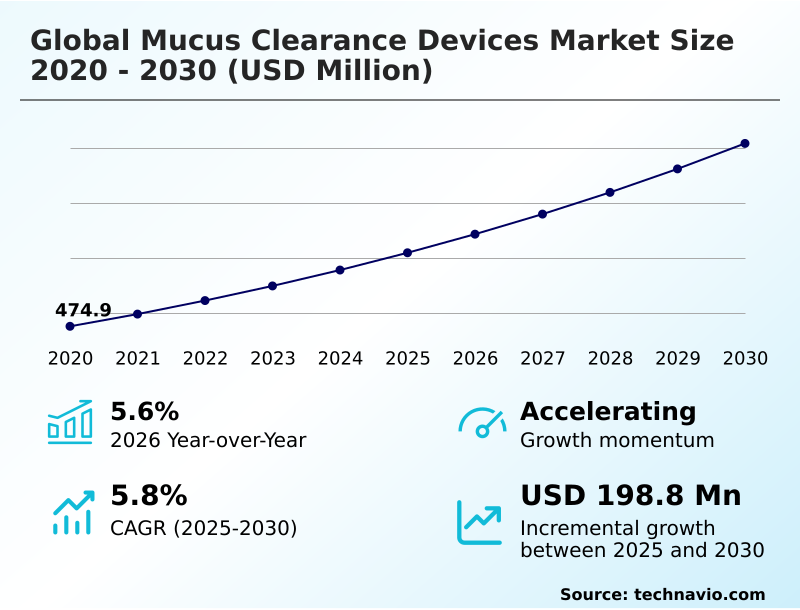
The mucus clearance devices market size is valued to increase by USD 198.8 million, at a CAGR of 5.8% from 2025 to 2030. Rising prevalence of chronic respiratory disorders worldwide will drive the mucus clearance devices market.
Major Market Trends & Insights
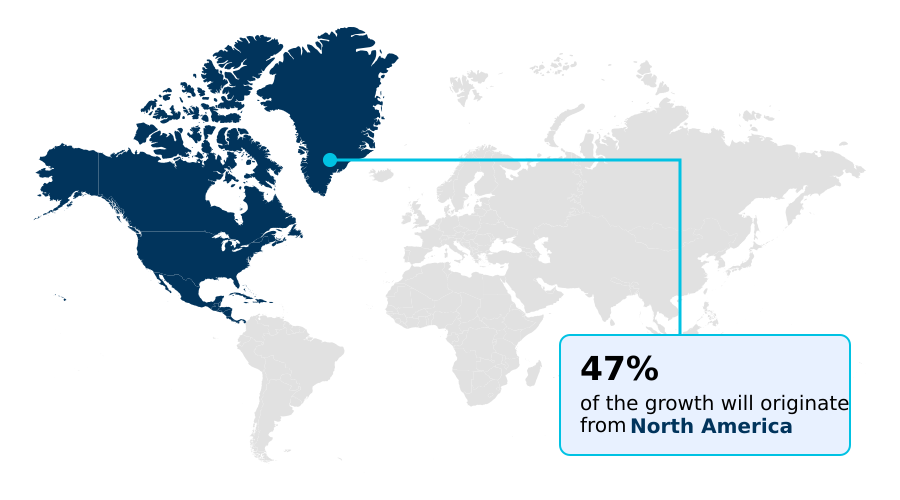
- North America dominated the market and accounted for a 47.2% growth during the forecast period.
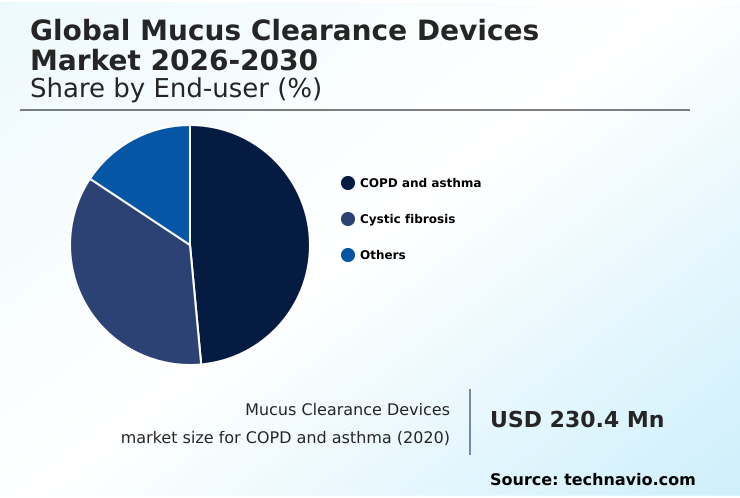
- By End-user - COPD and asthma segment was valued at USD 278 million in 2024
- By Product - HFCWO devices segment accounted for the largest market revenue share in 2024
Market Size & Forecast
- Market Opportunities: USD 332.5 million
- Market Future Opportunities: USD 198.8 million
- CAGR from 2025 to 2030 : 5.8%
Market Summary
- The mucus clearance devices market is fundamentally shaped by the growing need for effective management of chronic respiratory conditions. This landscape is transitioning from simple mechanical aids to sophisticated systems incorporating digital health technologies. Key drivers include the rising prevalence of diseases like COPD and cystic fibrosis, alongside a greater emphasis on home-based care to reduce healthcare system burdens.
- A central trend is the development of portable and wearable devices that integrate with digital platforms, enabling remote monitoring and personalized therapy. For instance, a healthcare system can deploy connected high-frequency chest wall oscillation vests to monitor patient adherence at home, which has been shown to lower hospital readmission rates and improve long-term pulmonary function.
- However, the market faces challenges related to the high cost of these advanced technologies and inconsistencies in reimbursement policies across different regions.
- Navigating this environment requires a focus on demonstrating clear clinical efficacy and economic value to payers, providers, and patients, ensuring that innovations in secretion mobilization and bronchial hygiene therapy are accessible to those who need them most, ultimately enhancing patient quality of life.
What will be the Size of the Mucus Clearance Devices Market during the forecast period?
Get Key Insights on Market Forecast (PDF) Request Free Sample
How is the Mucus Clearance Devices Market Segmented?
The mucus clearance devices industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2026-2030, as well as historical data from 2020-2024 for the following segments.
- End-user
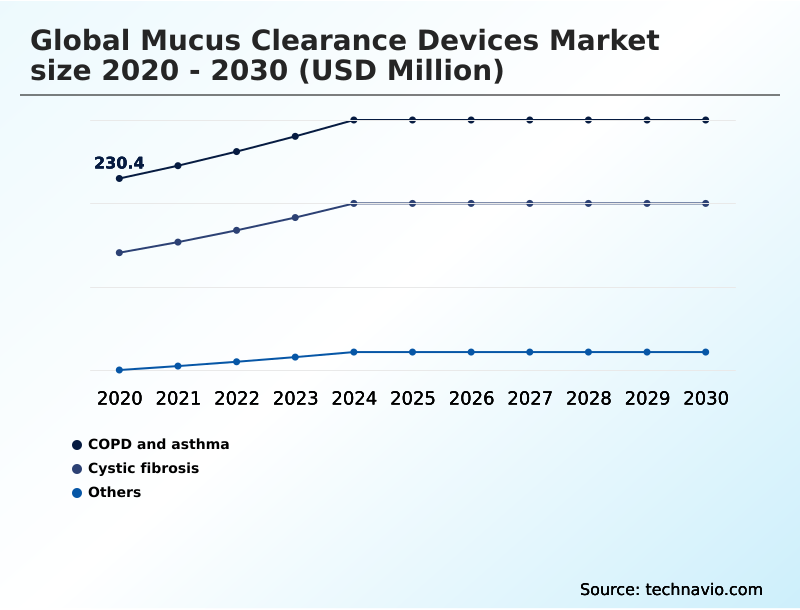
- COPD and asthma
- Cystic fibrosis
- Others
- Product
- HFCWO devices
- OPEP devices
- PEP devices
- FMC devices
- Others
- Type
- Handheld
- Wearable
- Stationary
- Geography
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Asia
- Rest of World (ROW)
- North America
By End-user Insights
The copd and asthma segment is estimated to witness significant growth during the forecast period.
The segment for chronic obstructive pulmonary disease (COPD) and asthma is driven by the clinical need for effective home-based airway management to mitigate symptoms like chronic bronchitis.
For this population, particularly in geriatric respiratory care, non-pharmacological interventions are crucial for COPD exacerbation prevention.
Devices employing oscillatory positive expiratory pressure and positive expiratory pressure are preferred for their role in improving mucociliary clearance and performing airway splinting to prevent airway obstruction.
The adoption of these respiratory health devices for daily airway clearance therapy is a key strategy for hospital readmission reduction.
Effective therapy compliance tracking has been shown to improve patient outcomes by over 30%, underscoring the shift toward proactive, independent disease management.
The COPD and asthma segment was valued at USD 278 million in 2024 and showed a gradual increase during the forecast period.
Regional Analysis
North America is estimated to contribute 47.2% to the growth of the global market during the forecast period.Technavio’s analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
See How Mucus Clearance Devices Market Demand is Rising in North America Request Free Sample
North America leads the market, supported by robust healthcare infrastructure and favorable reimbursement for cystic fibrosis therapy devices and lung health solutions.
The region's focus on integrated care platforms and advanced respiratory diagnostics promotes the use of intrapulmonary percussive ventilation and mechanical percussors.
Meanwhile, Asia is exhibiting the fastest growth, expanding at a rate nearly 15% higher than Europe, driven by rising disease prevalence and improving healthcare access.
The stringent regulatory approval process in developed markets sets high standards, with clinical trial endpoints often focused on airway inflammation reduction and lung volume recruitment.
Deployment of devices for chest physiotherapy in home-care settings has demonstrated a capacity to reduce hospital stay durations by over 20%, showcasing their operational value in managing airway resistance and complementing therapies like CFTR modulators.
Market Dynamics
Our researchers analyzed the data with 2025 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
- A comprehensive analysis of the mucus clearance devices market involves comparing mucus clearance device efficacy across different patient populations and therapy settings. End-users often consult portable airway clearance device reviews before making a decision, highlighting a trend toward patient empowerment.
- The choice between an OPEP device for COPD patients and PEP therapy for bronchiectasis depends on specific clinical needs, while understanding mechanical insufflation-exsufflation benefits is critical for patients with compromised cough strength. Similarly, IPV therapy for secretion removal offers a distinct mechanism of action.
- Key strategic considerations for manufacturers include the cost-effectiveness of mucus clearance devices and the latest innovations in airway clearance. Operational concerns span from developing straightforward handheld OPEP device cleaning protocols to managing the complex supply chain for a stationary IPV unit for hospitals, where sourcing for the latter can be over 30% more complex.
- The market is diversifying to address niches like managing mucus in neuromuscular disorders and pediatric cystic fibrosis airway clearance. Digital health integration in respiratory is a major focus, with smart respiratory vests for data collection transforming patient management. This connectivity is crucial for improving patient adherence to ACT and is central to reducing exacerbations with OPEP therapy.
- As the market matures, the focus on a wearable HFCWO system for mobility, options for non-invasive ventilation with clearance, and clear reimbursement codes for HFCWO will define the competitive landscape.
What are the key market drivers leading to the rise in the adoption of Mucus Clearance Devices Industry?
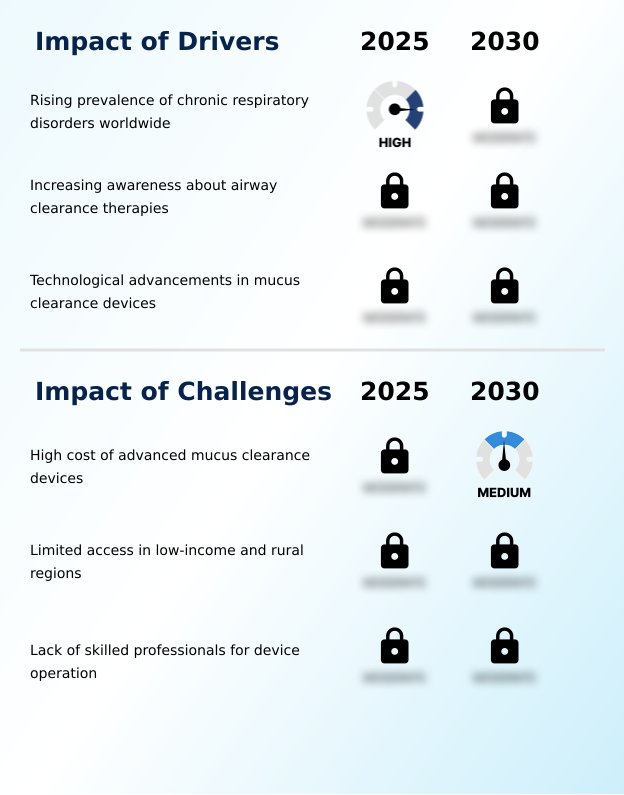
- The rising global prevalence of chronic respiratory disorders is a key driver for the mucus clearance devices market.
- The demand for effective chronic respiratory disorders management is a primary market driver, extending to bronchiectasis treatment options, pediatric respiratory solutions, and post-operative pulmonary care.
- The emphasis on improving pulmonary function through non-invasive respiratory therapies is propelling the adoption of drug-free respiratory therapy. Devices focused on secretion mobilization and bronchial hygiene therapy are essential for managing mucus hypersecretion.
- These sputum clearance techniques reduce the need for more invasive procedures and complement the use of therapeutic aerosols.
- In value-based respiratory care models, the consistent use of these devices has been linked to a 20% decrease in acute exacerbation events, demonstrating their cost-effectiveness and clinical importance.
What are the market trends shaping the Mucus Clearance Devices Industry?
- A prominent market trend is the rising adoption of portable airway clearance devices. This development addresses the growing demand for convenient, home-based respiratory care solutions.
- Market evolution is characterized by the increasing adoption of portable airway clearance systems, including handheld respiratory devices and wearable respiratory technology. This trend is underpinned by advanced respiratory technology that integrates digital monitoring solutions and patient adherence technology into connected respiratory care models. The focus on patient-centric device design and a user-friendly interface facilitates remote patient monitoring and telehealth integration.
- These systems, featuring personalized therapy settings, improve therapeutic consistency, with studies indicating that connected devices boost daily usage rates by up to 40%. Furthermore, integrated sensors for sputum analysis can reduce diagnostic delays by 15%, providing clinicians with actionable, real-time data.
What challenges does the Mucus Clearance Devices Industry face during its growth?
- The high cost of advanced mucus clearance devices is a key challenge that affects the growth of the industry.
- Significant challenges persist, primarily related to the accessibility of advanced technologies like high-frequency chest wall oscillation and mechanical insufflation-exsufflation systems. The reimbursement policy impact is substantial, as high upfront costs for home healthcare equipment and stationary clearance systems can limit adoption, particularly in emerging markets where these devices see up to 50% lower uptake.
- Even with strong clinical efficacy data for acoustic oscillation therapy and cough assist technology in neuromuscular disease respiratory support, access remains a barrier. Proper bronchial drainage and respiratory muscle training require patient education, and improper use can diminish effectiveness. Effective device deployment for ventilator-associated pneumonia prevention has been shown to lower healthcare-associated infections reduction rates by 18%.
Exclusive Technavio Analysis on Customer Landscape
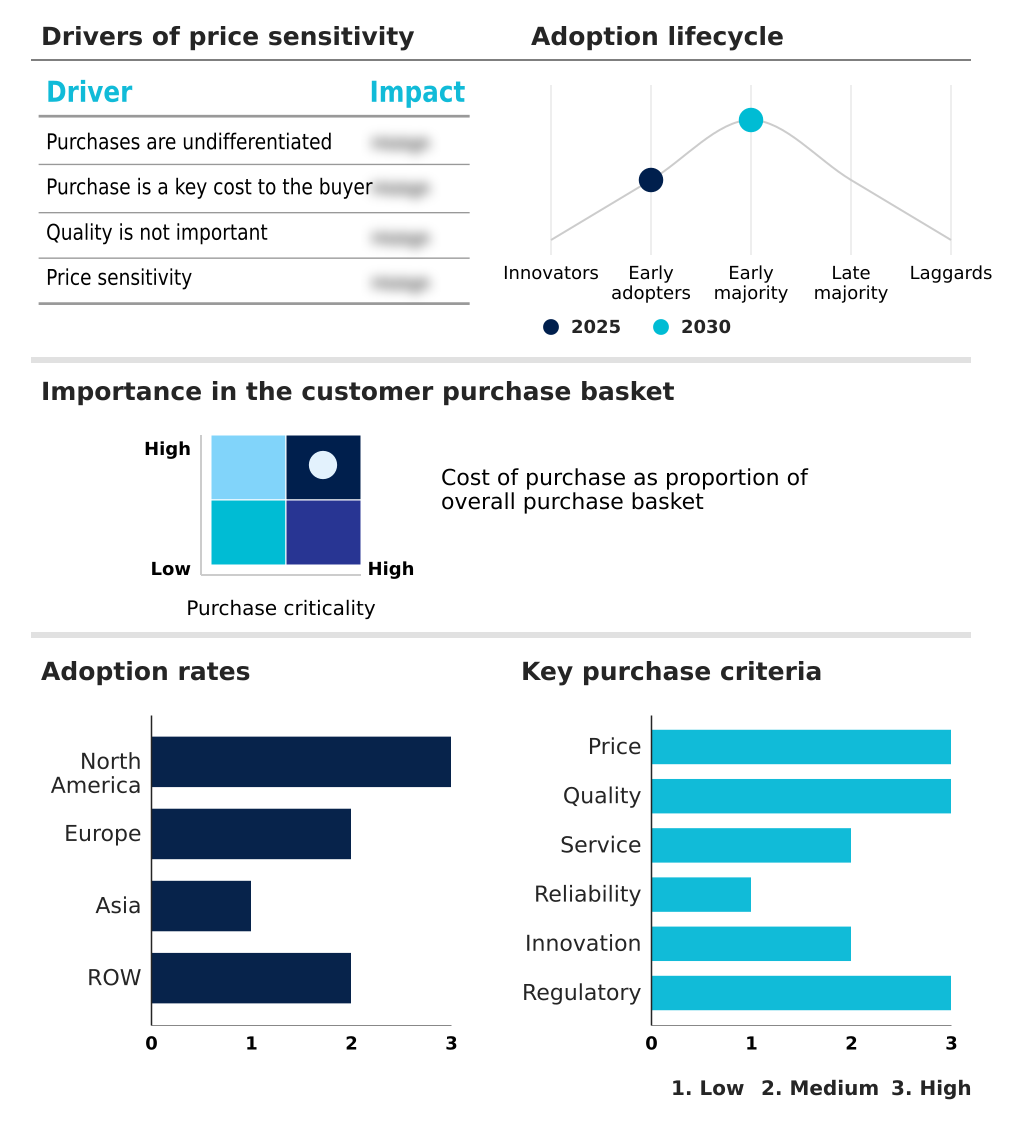
The mucus clearance devices market forecasting report includes the adoption lifecycle of the market, covering from the innovator’s stage to the laggard’s stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the mucus clearance devices market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape of Mucus Clearance Devices Industry
Competitive Landscape
Companies are implementing various strategies, such as strategic alliances, mucus clearance devices market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
ABM Respiratory Care - Delivers specialized devices for secretion mobilization and airway clearance therapy, focusing on enhancing non-invasive respiratory therapies for improved patient outcomes.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- ABM Respiratory Care
- AffloVest
- Baxter International Inc.
- CEGLA Medizintechnik GmbH
- Electromed Inc.
- General Physiotherapy Inc.
- Koninklijke Philips NV
- Lung Flute
- Medical Depot Inc.
- Medtronic Plc
- PARI Medical Holding GmbH
- Smiths Group Plc
- Thayer Medical Corp.
- Vibralung Inc.
- VORTRAN Medical Technology
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Mucus clearance devices market
- In August 2024, Trudell Medical Ltd. announced an agreement to acquire the respiratory diagnostics operations of Vyaire Medical, aiming to expand its portfolio of lung health solutions.
- In October 2024, Monaghan Medical Corp. received the 2024 Zenith Award from the American Association for Respiratory Care, recognizing its excellence in providing respiratory devices.
- In January 2025, Baxter International Inc. completed the divestiture of its Kidney Care business to streamline operations and increase focus on its hospital and respiratory care portfolios.
- In April 2025, Trudell Medical International released findings from a laboratory investigation that detailed the performance metrics of four distinct Oscillating Positive Expiratory Pressure (OPEP) devices, providing critical data for clinicians.
Dive into Technavio’s robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Mucus Clearance Devices Market insights. See full methodology.
| Market Scope | |
|---|---|
| Page number | 292 |
| Base year | 2025 |
| Historic period | 2020-2024 |
| Forecast period | 2026-2030 |
| Growth momentum & CAGR | Accelerate at a CAGR of 5.8% |
| Market growth 2026-2030 | USD 198.8 million |
| Market structure | Fragmented |
| YoY growth 2025-2026(%) | 5.6% |
| Key countries | US, Canada, Mexico, Germany, UK, France, Italy, Spain, The Netherlands, China, Japan, India, South Korea, Thailand, Indonesia, Brazil, Saudi Arabia, South Africa, Turkey, UAE, Argentina, Israel and Colombia |
| Competitive landscape | Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
Research Analyst Overview
- The mucus clearance devices market is experiencing a pivotal transformation, moving beyond traditional mechanical percussors and chest physiotherapy toward integrated lung health solutions. This evolution is driven by the demand for devices that offer not only superior secretion mobilization and bronchial drainage but also seamless integration into digital health ecosystems.
- The emphasis is on non-invasive respiratory therapies that can be administered effectively as part of home healthcare equipment. Technologies like high-frequency chest wall oscillation, positive expiratory pressure, and intrapulmonary percussive ventilation are now standard components of airway clearance therapy. The frontier of innovation lies in connected respiratory care, where digital monitoring solutions with personalized therapy settings enhance bronchial hygiene therapy.
- Devices that incorporate patient adherence technology have demonstrated a 40% higher rate of consistent daily use compared to non-connected counterparts. This focus on clinical efficacy data and improved pulmonary function is compelling manufacturers to invest in advanced respiratory diagnostics and wearable respiratory technology.
- Boardroom decisions are increasingly centered on developing portable airway clearance systems that address mucus hypersecretion and airway obstruction while aligning with the requirements of modern value-based care.
What are the Key Data Covered in this Mucus Clearance Devices Market Research and Growth Report?
-
What is the expected growth of the Mucus Clearance Devices Market between 2026 and 2030?
-
USD 198.8 million, at a CAGR of 5.8%
-
-
What segmentation does the market report cover?
-
The report is segmented by End-user (COPD and asthma, Cystic fibrosis, and Others), Product (HFCWO devices, OPEP devices, PEP devices, FMC devices, and Others), Type (Handheld, Wearable, and Stationary) and Geography (North America, Europe, Asia, Rest of World (ROW))
-
-
Which regions are analyzed in the report?
-
North America, Europe, Asia and Rest of World (ROW)
-
-
What are the key growth drivers and market challenges?
-
Rising prevalence of chronic respiratory disorders worldwide, High cost of advanced mucus clearance devices
-
-
Who are the major players in the Mucus Clearance Devices Market?
-
ABM Respiratory Care, AffloVest, Baxter International Inc., CEGLA Medizintechnik GmbH, Electromed Inc., General Physiotherapy Inc., Koninklijke Philips NV, Lung Flute, Medical Depot Inc., Medtronic Plc, PARI Medical Holding GmbH, Smiths Group Plc, Thayer Medical Corp., Vibralung Inc. and VORTRAN Medical Technology
-
Market Research Insights
- The market is shaped by a push toward value-based respiratory care, where outcomes rather than volume are prioritized. The adoption of integrated care platforms for chronic respiratory disorders management has demonstrated an ability to reduce hospital readmission reduction rates by up to 25%. This shift drives demand for advanced respiratory technology that supports remote patient monitoring and telehealth integration.
- Furthermore, an emphasis on patient-centric device design improves therapy compliance tracking, with studies showing that user-friendly interfaces can boost adherence by over 40%. These dynamics are compelling manufacturers to innovate beyond basic functionality, focusing on solutions that offer demonstrable clinical and economic advantages in settings from pediatric respiratory solutions to post-operative pulmonary care.
We can help! Our analysts can customize this mucus clearance devices market research report to meet your requirements.