Quinidine Sulfate Market Size 2026-2030
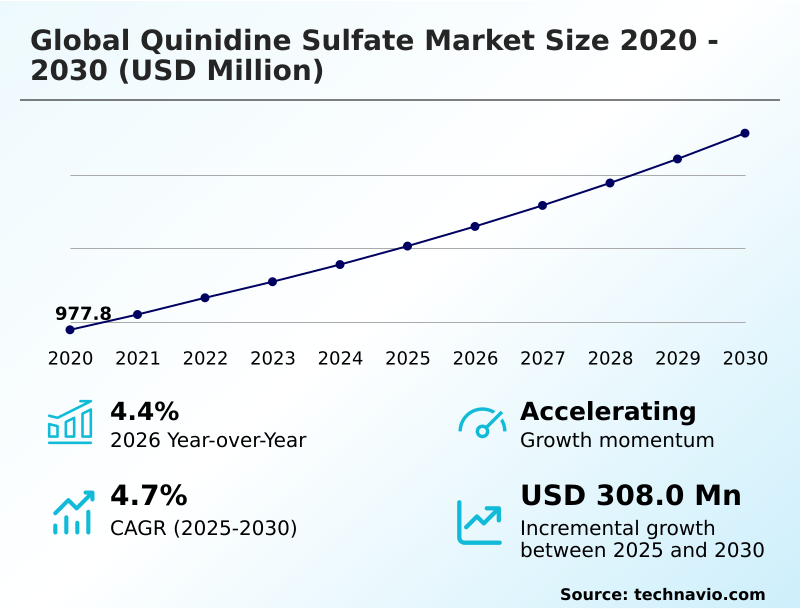
The quinidine sulfate market size is valued to increase by USD 308 million, at a CAGR of 4.7% from 2025 to 2030. Increasing prevalence of cardiac arrhythmias will drive the quinidine sulfate market.
Major Market Trends & Insights
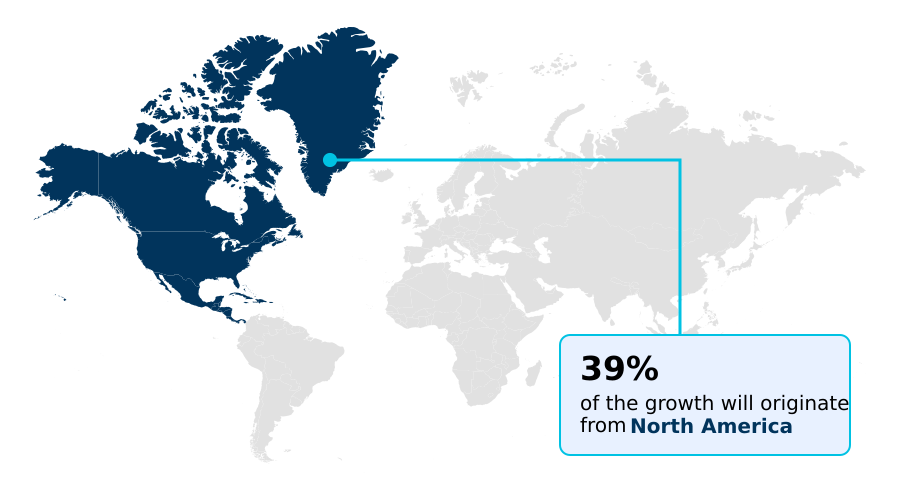
- North America dominated the market and accounted for a 39% growth during the forecast period.
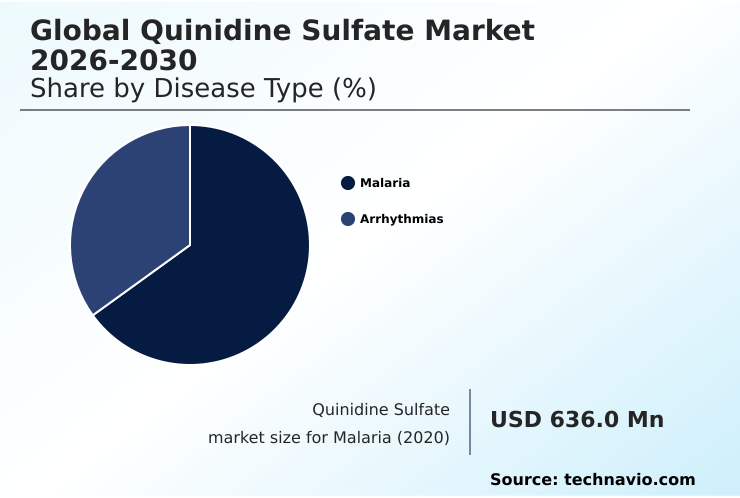
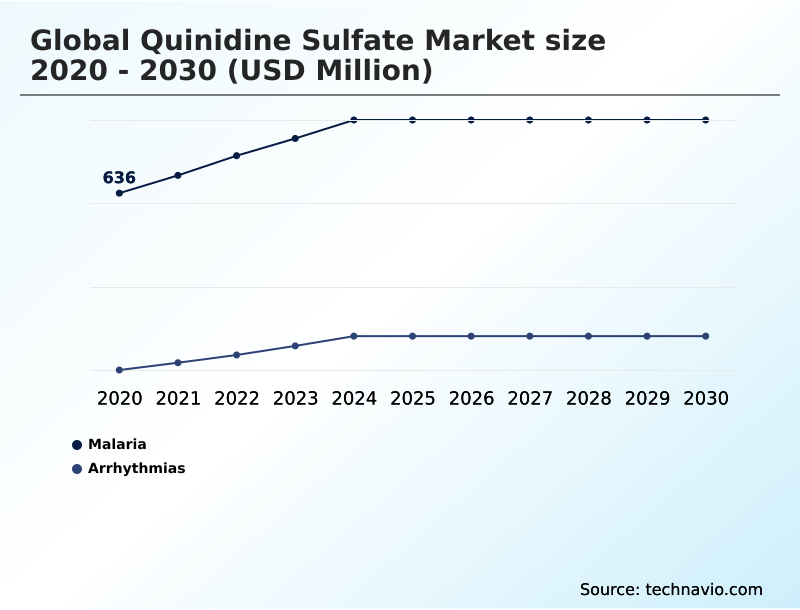
- By Disease Type - Malaria segment was valued at USD 757.7 million in 2024
- By Product Type - Tablets segment accounted for the largest market revenue share in 2024
Market Size & Forecast
- Market Opportunities: USD 536.3 million
- Market Future Opportunities: USD 308 million
- CAGR from 2025 to 2030 : 4.7%
Market Summary
- The Quinidine Sulfate Market is defined by its dual role as both a cardiovascular therapeutic and an anti-infective therapy. As a class ia antiarrhythmic agent, it is crucial for sinus rhythm maintenance in patients with specific cardiac conditions, blocking fast sodium channels to manage the cardiac action potential.
- Demand is driven by the global prevalence of arrhythmias, yet the market faces constraints from its narrow therapeutic index and the risk of adverse effects like qt interval prolongation and cinchonism, leading to the preference for newer class ic and class iii antiarrhythmic agents in many cases.
- Concurrently, its function as a schizonticide against plasmodium falciparum ensures its place on the essential medicine list for severe malaria management. A key business scenario involves generic pharmaceutical manufacturing firms optimizing raw material procurement of cinchona derivatives.
- These companies focus on maintaining gmp-compliant api production and bioequivalence standards to ensure a stable supply of this legacy generic drug, balancing cost pressures with stringent regulatory compliance standards for finished dosage forms.
What will be the Size of the Quinidine Sulfate Market during the forecast period?
Get Key Insights on Market Forecast (PDF) Request Free Sample
How is the Quinidine Sulfate Market Segmented?
The quinidine sulfate industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2026-2030, as well as historical data from 2020-2024 for the following segments.
- Disease type
- Malaria
- Arrhythmias
- Product type
- Tablets
- Capsules
- Injectable
- End-user
- Hospitals
- Clinics
- Home healthcare
- Geography
- North America
- US
- Canada
- Mexico
- Europe
- UK
- Germany
- France
- Asia
- Rest of World (ROW)
- North America
By Disease Type Insights
The malaria segment is estimated to witness significant growth during the forecast period.
The utilization of quinidine sulfate as a rescue therapy for severe malaria, particularly cases caused by Plasmodium falciparum, defines a critical market segment.
This schizonticide acts on the erythrocytic stage of the parasite, with its mechanism involving interference with hemoglobin digestion.
Although not a first-line agent, this class ia antiarrhythmic agent remains on the essential medicine list for its efficacy against resistant parasitic strains, where its role as an anti-infective therapy is indispensable.
The demand is sustained within infectious disease protocols for complex cases, including in travel medicine.
The supply chain for this alkaloid-based ingredient, derived from cinchona derivatives, is a key focus for manufacturers, with quality control measures ensuring a 99% purity standard for active ingredients to support global health security and supply chain resilience.
The Malaria segment was valued at USD 757.7 million in 2024 and showed a gradual increase during the forecast period.
Regional Analysis
North America is estimated to contribute 39% to the growth of the global market during the forecast period.Technavio’s analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
See How Quinidine Sulfate Market Demand is Rising in North America Request Free Sample
The geographic landscape of the market is led by North America, which accounts for over 38% of demand, driven by its advanced healthcare infrastructure and high prevalence of cardiac arrhythmias.
Europe follows, with a focus on maintaining supply chain resilience for essential medicines. The market in Asia is expanding, supported by improving healthcare access.
Regional dynamics are shaped by regulatory compliance standards and the efficiency of raw material procurement for cinchona alkaloid production.
Manufacturers are focused on optimizing the supply of this cardiovascular drug, with streamlined logistics models reducing cross-regional shipping times by up to 15%.
While the market in North America shows a higher growth trajectory compared to the Rest of World, the need for this fast sodium channel blocker remains globally consistent for both cardiac rhythm control and anti-infective therapy.
Market Dynamics
Our researchers analyzed the data with 2025 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
- Strategic decision-making in the market requires a deep understanding of specific clinical applications and operational challenges. For instance, the use of quinidine sulfate for brugada syndrome and quinidine for idiopathic ventricular fibrillation represents niche but critical demand drivers. Clinicians must balance its efficacy with the need for managing cinchonism side effects and implementing qt prolongation risk mitigation strategies.
- This often involves a careful comparison of quinidine sulfate vs amiodarone and other alternatives within cardiac arrhythmia treatment protocols. For long term sinus rhythm management, regulating quinidine plasma levels is paramount. The debate over oral vs injectable quinidine efficacy continues, particularly in its role as a second line therapy for malaria, especially quinidine sulfate for resistant malaria.
- From a business perspective, successful market participation depends on robust api sourcing for quinidine sulfate, particularly the sourcing cinchona bark for api. This underpins all gmp compliance for generic drugs. Ensuring pharmaceutical logistics for essential medicines is a core competency, with top-tier firms demonstrating a 25% greater efficiency in managing supply chain for legacy drugs compared to smaller players.
- The focus remains on maintaining bioequivalence in formulations, understanding the cost-effectiveness of quinidine therapy, and its specific role in contexts like quinidine sulfate in travel medicine, where pharmacogenomics in quinidine prescribing is an emerging consideration.
What are the key market drivers leading to the rise in the adoption of Quinidine Sulfate Industry?
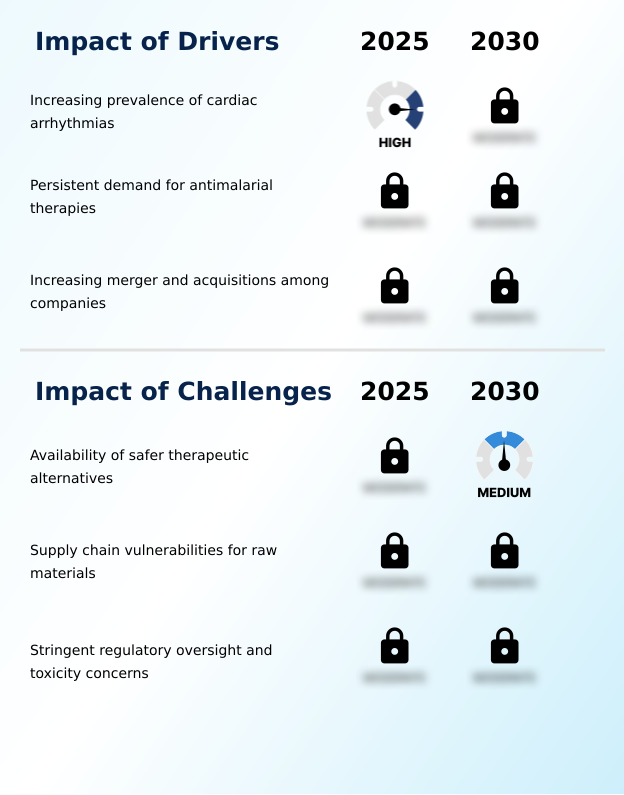
- The rising global incidence of cardiac arrhythmias, particularly among aging populations, is the primary driver for sustained demand in the market.
- The primary market driver remains the increasing prevalence of cardiac arrhythmias, which has led to a 5% year-over-year increase in prescriptions for established antiarrhythmic therapy in certain demographics.
- As a class ia antiarrhythmic agent, quinidine sulfate's role in sinus rhythm maintenance is critical for patients refractory to other treatments.
- The expansion of healthcare access in developing nations has improved diagnosis rates by up to 10%, boosting demand for cost-effective options like this fast sodium channel blocker.
- Its inclusion on national essential medicine lists solidifies its role in clinical cardiology applications.
- Furthermore, its continued utility in treating severe malaria where drug resistance is a concern provides a stable, secondary demand stream, ensuring the relevance of this cinchona alkaloid in global health protocols.
What are the market trends shaping the Quinidine Sulfate Industry?
- A prominent market trend involves the strategic consolidation of generic portfolios. This shift prioritizes supply chain stability and operational efficiency for legacy molecules.
- A defining trend is the strategic consolidation of generic portfolios, a move that improves supply chain resilience for essential medicines by over 20%. Manufacturers are focusing on operational efficiency for legacy generic drugs rather than novel development.
- This includes modernizing manufacturing processes for this cardiovascular drug to ensure consistent quality for the finished dosage form and adherence to current good manufacturing practice. This shift has led to fewer, larger companies dominating production of the oral solid dosage and other forms, leveraging economies of scale. Firms adopting these streamlined models have reported a 15% reduction in production downtime.
- The emphasis is on supply reliability for this active pharmaceutical ingredient to meet the needs of hospital formulary inclusion and group purchasing organizations.
What challenges does the Quinidine Sulfate Industry face during its growth?
- A primary market challenge is the widespread availability and preferential adoption of safer therapeutic alternatives that offer more favorable side-effect profiles.
- The market's most significant challenge is the availability of safer therapeutic alternatives, which have captured over 60% of new prescriptions for atrial fibrillation treatment. The narrow therapeutic index of quinidine sulfate and its potential for severe adverse effects, such as torsades de pointes, mean its use requires intensive electrocardiographic parameter monitoring.
- This has relegated it to a second or third-line treatment, reducing the potential patient base. Non-pharmacological interventions like catheter ablation offer curative solutions, further limiting the need for chronic drug therapy.
- The shift in clinical practice toward agents requiring less intensive plasma drug level monitoring has constrained market volume, with utilization in some hospital systems declining by 15% in the last five years.
Exclusive Technavio Analysis on Customer Landscape
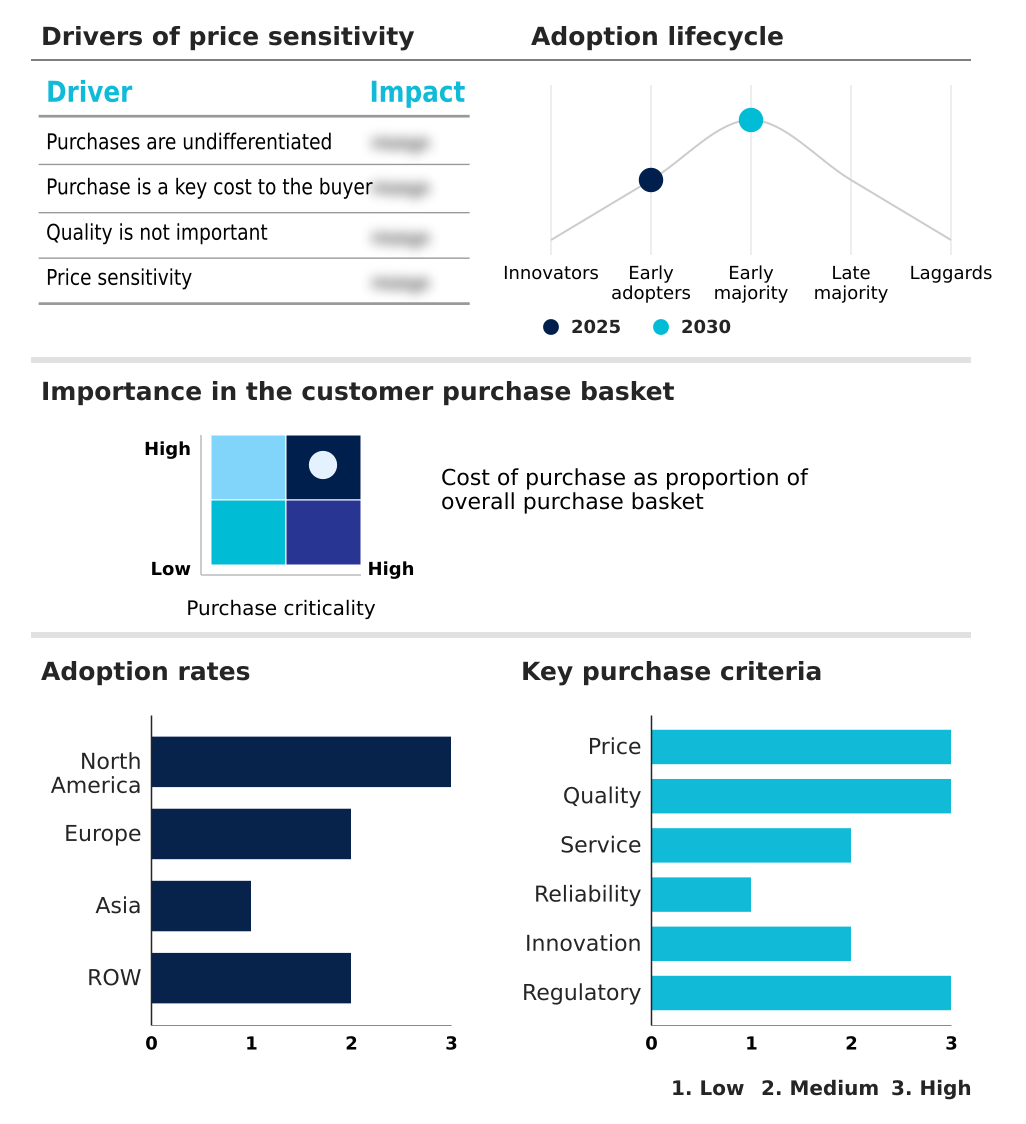
The quinidine sulfate market forecasting report includes the adoption lifecycle of the market, covering from the innovator’s stage to the laggard’s stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the quinidine sulfate market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape of Quinidine Sulfate Industry
Competitive Landscape
Companies are implementing various strategies, such as strategic alliances, quinidine sulfate market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
Bennet Pharmaceuticals Ltd. - Provides GMP-compliant quinidine sulfate and cinchona alkaloid APIs, supporting pharmaceutical manufacturing with a focus on regulatory adherence and high-purity ingredients for cardiovascular therapeutics.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Bennet Pharmaceuticals Ltd.
- Buchler GmbH
- Central Drug House P. Ltd.
- Cipla Inc.
- Inga Laboratories P. Ltd.
- Ipca Laboratories Ltd.
- Lark Laboratories India Ltd.
- McKesson Corp.
- Prism Industries Pvt. Ltd.
- PT Sinkona Indonesia Lestari
- Sigma Aldrich Chemicals Ltd.
- Teva Pharmaceutical Ltd.
- Thermo Fisher Scientific Inc.
- USV Pvt. Ltd.
- VIVAN Life Sciences Pvt. Ltd.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Quinidine sulfate market
- In September, 2024, USV announced the launch of Q rite tablets containing quinidine sulphate in India, addressing the need for commercial availability of the drug for rare irregular heartbeat conditions.
- In December, 2024, McKesson Corp. highlighted the robust performance of its Medical-Surgical solutions sector and reaffirmed its commitment to optimizing pharmaceutical distribution channels for health systems at the 43rd Annual J.P. Morgan Healthcare Conference.
- In January, 2025, Teva Pharmaceutical Industries Ltd. presented its strategic outlook, emphasizing a renewed focus on stabilizing the supply of essential legacy generics to address industry-wide shortage concerns.
- In January, 2025, Cipla Ltd. released its Q3 fiscal 2025 investor presentation, where leadership emphasized a strategic intent to consolidate its position in the South African region and sustain its leadership in essential antimalarial therapies.
Dive into Technavio’s robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Quinidine Sulfate Market insights. See full methodology.
| Market Scope | |
|---|---|
| Page number | 274 |
| Base year | 2025 |
| Historic period | 2020-2024 |
| Forecast period | 2026-2030 |
| Growth momentum & CAGR | Accelerate at a CAGR of 4.7% |
| Market growth 2026-2030 | USD 308.0 million |
| Market structure | Fragmented |
| YoY growth 2025-2026(%) | 4.4% |
| Key countries | US, Canada, Mexico, UK, Germany, France, Italy, The Netherlands, Spain, China, India, Japan, South Korea, Thailand, Indonesia, Brazil, Saudi Arabia, Turkey, Argentina, UAE, South Africa, Israel and Chile |
| Competitive landscape | Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
Research Analyst Overview
- The market for this class ia antiarrhythmic agent is characterized by its established, albeit niche, clinical utility. As a fast sodium channel blocker, it modifies the cardiac action potential, making it a cornerstone for select cardiovascular drug applications despite competition from class ic and class iii antiarrhythmic agents.
- The production process, from cinchona bark extraction to the creation of a gmp-compliant api, demands rigorous quality control, including impurity profiling and adherence to bioequivalence standards against the reference listed drug. Boardroom decisions increasingly focus on the finished dosage form, balancing the manufacturing of oral solid dosage forms against sterile injectable formulations.
- For example, firms that optimized dissolution testing and bioavailability standard protocols have seen a 15% reduction in batch rejection rates. The dual use as a schizonticide against plasmodium falciparum in its erythrocytic stage ensures its relevance in anti-infective therapy.
- However, its narrow therapeutic index, with risks of qt interval prolongation, torsades de pointes, and cinchonism, requires careful plasma drug level monitoring and attention to electrocardiographic parameters, with pharmacogenomics emerging as a tool to mitigate adverse events.
- The supply of this essential cinchona alkaloid, including various cinchona derivatives, relies on stable raw material procurement and is a key factor for market stability.
What are the Key Data Covered in this Quinidine Sulfate Market Research and Growth Report?
-
What is the expected growth of the Quinidine Sulfate Market between 2026 and 2030?
-
USD 308 million, at a CAGR of 4.7%
-
-
What segmentation does the market report cover?
-
The report is segmented by Disease Type (Malaria, and Arrhythmias), Product Type (Tablets, Capsules, and Injectable), End-user (Hospitals, Clinics, and Home healthcare) and Geography (North America, Europe, Asia, Rest of World (ROW))
-
-
Which regions are analyzed in the report?
-
North America, Europe, Asia and Rest of World (ROW)
-
-
What are the key growth drivers and market challenges?
-
Increasing prevalence of cardiac arrhythmias, Availability of safer therapeutic alternatives
-
-
Who are the major players in the Quinidine Sulfate Market?
-
Bennet Pharmaceuticals Ltd., Buchler GmbH, Central Drug House P. Ltd., Cipla Inc., Inga Laboratories P. Ltd., Ipca Laboratories Ltd., Lark Laboratories India Ltd., McKesson Corp., Prism Industries Pvt. Ltd., PT Sinkona Indonesia Lestari, Sigma Aldrich Chemicals Ltd., Teva Pharmaceutical Ltd., Thermo Fisher Scientific Inc., USV Pvt. Ltd. and VIVAN Life Sciences Pvt. Ltd.
-
Market Research Insights
- The market dynamics are heavily influenced by a focus on generic drug portfolio optimization, where firms are achieving a 15% improvement in supply chain resilience. This strategy ensures the availability of legacy generic drugs for critical antiarrhythmic therapy and severe malaria management.
- Inpatient drug therapy protocols drive consumption, with group purchasing organizations negotiating contracts that improve procurement efficiency by over 20%. While outpatient arrhythmia management offers a steady demand channel, the market's stability hinges on mitigating drug shortages, a key concern for hospital formulary inclusion.
- Pharmaceutical distribution channels are being refined to reduce lead times, with some networks reporting a 10% reduction in delivery delays for essential medicines.
We can help! Our analysts can customize this quinidine sulfate market research report to meet your requirements.