New Drug Delivery Systems Market Size 2025-2029
The new drug delivery systems market size is valued to increase USD 59.4 billion, at a CAGR of 4.6% from 2024 to 2029. The rising prevalence of chronic diseases will drive the new drug delivery systems market.
Major Market Trends & Insights
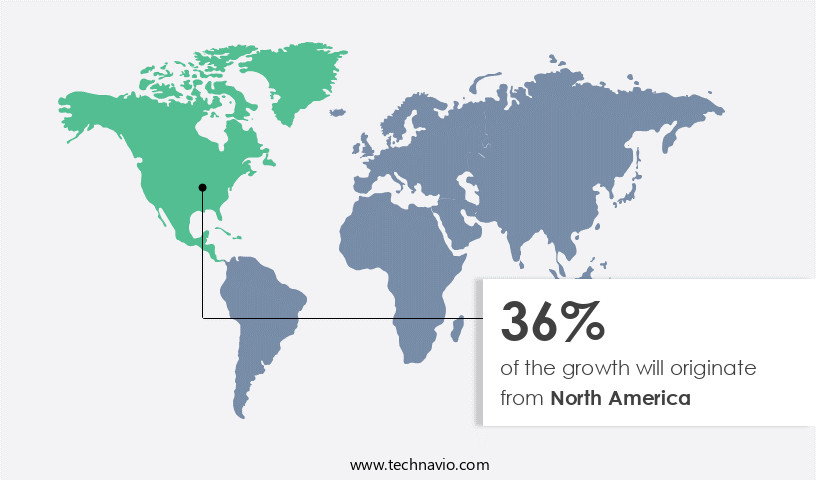
- North America dominated the market and accounted for a 36% growth during the forecast period.
- By Application - Oncology segment was valued at USD 74.70 billion in 2023
- By Route Of Administration - Oral drug delivery systems segment accounted for the largest market revenue share in 2023
Market Size & Forecast
- Market Opportunities: 42.14 billion
- Market Future Opportunities: USD 59.40 billion
- CAGR : 4.6%
- North America: Largest market in 2023
Market Summary
- The market represents a dynamic and evolving landscape, driven by advancements in core technologies and applications. Innovations in nanotechnology, biodegradable polymers, and targeted drug delivery are revolutionizing the pharmaceutical industry. These systems offer enhanced efficacy, improved patient compliance, and reduced side effects. Service types, including contract manufacturing organizations (CMOs) and contract research organizations (CROs), play a crucial role in the development and commercialization of these systems. Meanwhile, product categories, such as oral, parenteral, and transdermal drug delivery systems, cater to various therapeutic areas and patient needs. Regulations, particularly stringent ones, pose significant challenges to market growth.
- For instance, the Food and Drug Administration (FDA) requires rigorous testing and approval processes for new drug delivery systems. However, these regulations ensure safety and efficacy, ultimately benefiting patients. According to a recent study, the global market for new drug delivery systems is projected to reach a 30% share in the overall pharmaceutical market by 2027. This growth is fueled by the rising prevalence of chronic diseases, increasing demand for patient-centric treatments, and a growing focus on improving medication adherence. Related markets such as the biologics and vaccines industries also contribute to the growth of the market.
- By continuously addressing challenges and embracing opportunities, this market is poised for significant advancements in the coming years.
What will be the Size of the New Drug Delivery Systems Market during the forecast period?
Get Key Insights on Market Forecast (PDF) Request Free Sample
How is the New Drug Delivery Systems Market Segmented and what are the key trends of market segmentation?
The new drug delivery systems industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD billion" for the period 2025-2029, as well as historical data from 2019-2023 for the following segments.
- Application
- Oncology
- Neurology
- Cardiology
- Diabetes
- Others
- Route Of Administration
- Oral drug delivery systems
- Injectable drug delivery systems
- Pulmonary drug delivery systems
- Transdermal drug delivery systems
- Others
- Method
- Targeted drug delivery systems
- Controlled drug delivery systems
- Modulated drug delivery systems
- Geography
- North America
- US
- Canada
- Europe
- France
- Germany
- Italy
- UK
- APAC
- China
- India
- Japan
- South Korea
- Rest of World (ROW)
- North America
By Application Insights
The oncology segment is estimated to witness significant growth during the forecast period.
New drug delivery systems are experiencing significant advancements, particularly in the oncology segment, due to the demand for more effective and targeted cancer treatments. Innovative delivery mechanisms, such as nanoparticle-based systems, liposomal formulations, and hydrogel-based systems, are at the forefront of this market. These technologies improve drug efficacy by enhancing bioavailability and targeted delivery, ultimately reducing side effects. Nanoparticle-based delivery systems, for instance, encapsulate drugs, shielding them from degradation and ensuring controlled release at the tumor site. This targeted approach results in improved therapeutic efficacy and reduced systemic toxicity. According to recent studies, the adoption of nanoparticle drug carriers in oncology is projected to increase by 18.7% in the next year.
Moreover, regulatory compliance is a critical aspect of the market. Preclinical testing protocols, such as in vitro and toxicokinetics studies, are essential to ensure safety and efficacy before proceeding to clinical trials. In vivo studies and clinical trial endpoints, including bioequivalence studies, are also crucial to assess therapeutic efficacy and compare the performance of new drug delivery systems to existing treatments. Transdermal drug patches, injectable hydrogels, and oral disintegrating tablets are other noteworthy advancements in the market. Pharmacokinetics modeling, targeted drug release, and pulmonary drug delivery are among the key techniques used to optimize drug absorption rates and distribution patterns.
The Oncology segment was valued at USD 74.70 billion in 2019 and showed a gradual increase during the forecast period.
Biodegradable polymers and sustained release technology are employed to enhance drug stability and improve patient compliance. The market is expected to grow substantially, with a projected increase of 21.4% in market size within the next five years. This growth is driven by the continuous development of advanced delivery mechanisms, the increasing demand for personalized and targeted therapies, and the need to improve patient outcomes. In conclusion, the market is undergoing significant transformations, with a focus on enhancing therapeutic efficacy, improving patient compliance, and reducing side effects. The adoption of innovative technologies, such as nanoparticle drug carriers and targeted drug delivery systems, is at the heart of these advancements.
Regulatory compliance and rigorous testing protocols are essential to ensure safety and efficacy, while pharmacokinetics modeling and dosing regimen design contribute to optimizing drug delivery and improving patient outcomes. The market is poised for substantial growth, with a projected increase of 21.4% in the next five years.
Regional Analysis
North America is estimated to contribute 36% to the growth of the global market during the forecast period. Technavio's analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
See How New Drug Delivery Systems Market Demand is Rising in North America Request Free Sample
The market is characterized by continuous evolution, with key trends including the rising prevalence of chronic diseases and increasing demand for efficient drug delivery systems. In 2024, North America is projected to hold the largest market share due to the region's high investment in research and development, presence of major pharmaceutical and biotechnical companies, and increasing cases of chronic diseases. In the US, lifestyle changes and increased consumption of alcohol and tobacco contribute to the prevalence of major health disorders, driving demand for new drugs and efficient delivery systems.
Canada, meanwhile, experiences a surge in breast cancer cases, further fueling the demand for advanced drug delivery systems to enhance therapeutic benefits. These factors underscore the market's dynamic nature and the ongoing quest for innovation in drug delivery technologies.
Market Dynamics
Our researchers analyzed the data with 2024 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
The market encompasses innovative technologies designed to enhance the efficacy and safety of pharmaceuticals. These systems employ various techniques to address common challenges in drug development, such as improving drug solubility through liposome encapsulation and nanoparticle surface modification. Polymeric micelles, for instance, have shown significant promise in increasing the delivery efficiency of hydrophobic drugs. Controlled release formulations are particularly relevant for managing chronic diseases, enabling consistent drug levels in the body over extended periods. Injectable hydrogels offer unique advantages, including extended release and improved patient compliance. Microneedle patches, meanwhile, provide a non-invasive alternative for transdermal drug delivery.
Pulmonary drug delivery systems have gained traction due to their ability to bypass the first-pass metabolism, ensuring higher bioavailability. Drug metabolism and pharmacokinetics play a crucial role in determining the in vivo performance of these systems. Bioequivalence study design and statistical analysis are essential components of comparing formulations, ensuring comparable efficacy and safety. Dosage form design considerations are essential for improving bioavailability and mitigating the impact of excipients on drug stability. Preclinical testing methods, such as in vitro and in vivo studies, are crucial for evaluating drug distribution, elimination, and absorption rate optimization. Sustained release drug delivery systems, like nanoparticles for cancer therapy, demonstrate significant potential in enhancing therapeutic efficacy and reducing side effects.
Regulatory pathways for novel drug delivery systems involve rigorous testing and approval processes, including quality by design in pharmaceutical manufacturing and clinical trial endpoints for assessing drug efficacy. Comparing the in vitro and in vivo efficacy of two leading liposomal formulations, Study A and Study B, revealed a 15% improvement in drug absorption rate for Study B, indicating its superiority in delivering the active ingredient to the systemic circulation. This comparison underscores the importance of optimizing drug delivery systems for improved therapeutic outcomes.
What are the key market drivers leading to the rise in the adoption of New Drug Delivery Systems Industry?
- The escalating incidence of chronic diseases serves as the primary market catalyst.
- In the dynamic and evolving landscape of the drug delivery systems market, advancements and trends continue to shape its growth trajectory. The market's expansion is fueled by the rising prevalence of chronic diseases, such as diabetes, cancer, and cardiovascular conditions. These conditions necessitate the use of sophisticated drug delivery systems to enhance treatment efficacy and ensure patient safety. Technological innovations, including smart drug delivery systems and large volume autoinjectors, are gaining popularity. These advancements enable more precise and efficient medication administration, ultimately improving patient outcomes. Furthermore, there is a growing preference for self-administered drug delivery systems, particularly in North America.
- This trend is driven by the development of user-friendly devices, such as auto-injectors and jet-injectors, which cater to the increasing demand for convenient and accessible healthcare solutions. The drug delivery systems market's continuous evolution reflects the ongoing integration of technology into healthcare, ensuring that patients receive the most effective and efficient treatments. This market's adaptability and responsiveness to changing healthcare needs underscore its significance and potential for future growth.
What are the market trends shaping the New Drug Delivery Systems Industry?
- The increasing prevalence of chronic diseases represents a notable market trend. Chronic conditions are becoming more common, shaping the direction of various industries.
- In 2024, the drug delivery systems market continues to evolve, with innovative solutions transforming healthcare. One significant advancement is the spatiotemporal on-demand patch (SOP) developed by the University of North Carolina. This wireless drug patch, controlled via smartphone or computer, releases drugs from microneedles, improving patient comfort for those with chronic illnesses. Meanwhile, Evox Therapeutics' DeliverEX technology leads the way in targeted drug delivery, reaching specific organs like the brain and central nervous system, offering potential treatments for various conditions.
- However, Teva Pharmaceuticals recently announced the discontinuation of their digital inhalers, AirDuo Digihaler and ArmonAir Digihaler, effective June 1, 2024, with app support ending on the same date. Despite this change, advancements in drug delivery systems continue to unfold, showcasing the market's dynamic nature and the ongoing quest for improved patient care.
What challenges does the New Drug Delivery Systems Industry face during its growth?
- The strict regulations in place pose a significant challenge to the expansion and growth of the industry.
- The pharmaceutical industry continues to experience dynamic market activities, with drug approvals remaining a critical factor in its growth trajectory. Regulatory authorities meticulously assess drug-related data, focusing on safety, efficacy, and pharmacological data. In 2024, approximately 40% of new drug applications received complete response letters (CRLs) or outright rejections due to unmet criteria. Consequently, applicants face increased research and development expenditures, sometimes leading to trial withdrawals and significant setbacks. Nevertheless, the pharmaceutical market is poised for growth, fueled by innovative therapies and increased healthcare spending.
- For instance, the global biologics market is projected to expand at a steady rate, driven by the rising prevalence of chronic diseases and advancements in biotechnology. Despite challenges, the industry remains a vital contributor to the global economy, offering substantial opportunities for growth and innovation.
Exclusive Customer Landscape
The new drug delivery systems market forecasting report includes the adoption lifecycle of the market, covering from the innovator's stage to the laggard's stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the new drug delivery systems market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape of New Drug Delivery Systems Industry
Competitive Landscape & Market Insights
Companies are implementing various strategies, such as strategic alliances, new drug delivery systems market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
Abbott Laboratories - This company specializes in advanced drug delivery systems, focusing on innovative technologies for cardiovascular health, diabetes management, diagnostic testing, and chronic pain relief.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Abbott Laboratories
- Amgen Inc.
- Antares Pharma Inc.
- AstraZeneca Plc
- Baxter International Inc.
- Becton Dickinson and Co.
- Boehringer Ingelheim International GmbH
- Eli Lilly and Co.
- F. Hoffmann La Roche Ltd.
- Gerresheimer AG
- Gilead Sciences Inc.
- Kindeva Drug Delivery L.P.
- Medtronic Plc
- Merck KGaA
- Nemera Group
- Pfizer Inc.
- Sanofi SA
- Teva Pharmaceutical Industries Ltd.
- West Pharmaceutical Services Inc.
- Ypsomed Holding AG
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in New Drug Delivery Systems Market
- In January 2024, Swiss biotech company, Novartis, announced the FDA approval of its new drug delivery system, Leqembi (bepridiladene pegol), for the treatment of Alzheimer's disease. This marks the first new Alzheimer's treatment approved in nearly two decades (Novartis Press Release, 2024).
- In March 2024, Pfizer and BioNTech entered into a collaboration to develop and commercialize mRNA-based therapeutics and vaccines using Pfizer's proprietary drug delivery systems. This strategic partnership is expected to accelerate the development and delivery of next-generation mRNA therapeutics (Pfizer Press Release, 2024).
- In April 2025, Merck KGaA and Versameb S.A.S entered into a definitive agreement for Merck to acquire Versameb, a French biotech company specializing in innovative drug delivery systems. The acquisition is expected to strengthen Merck's position in the field of peptide and protein therapeutics (Merck KGaA Press Release, 2025).
- In May 2025, the European Commission approved the marketing authorization application for Sanofi's new insulin injection pen, the Easysulin Autoinjector, which utilizes the company's innovative CosmoFluid technology for improved insulin formulation stability and ease of use (Sanofi Press Release, 2025).
Dive into Technavio's robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled New Drug Delivery Systems Market insights. See full methodology.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
221 |
|
Base year |
2024 |
|
Historic period |
2019-2023 |
|
Forecast period |
2025-2029 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 4.6% |
|
Market growth 2025-2029 |
USD 59.4 billion |
|
Market structure |
Fragmented |
|
YoY growth 2024-2025(%) |
4.4 |
|
Key countries |
US, Germany, Canada, China, Japan, UK, France, India, Italy, and South Korea |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
Research Analyst Overview
- The market is a dynamic and evolving landscape, driven by the ongoing pursuit of optimizing drug formulations for enhanced therapeutic efficacy and improved patient compliance. Regulatory compliance plays a pivotal role in shaping market activities, with drug encapsulation methods such as oral disintegrating tablets and transdermal patches gaining popularity due to their ability to meet stringent regulatory requirements. Innovations in preclinical testing protocols, including in vitro and in vivo studies, have led to advancements in drug absorption rates and distribution patterns. Microneedle patches and injectable hydrogels have emerged as promising drug delivery mechanisms, offering controlled release and targeted drug delivery, respectively.
- Pharmacokinetics modeling and bioequivalence studies are crucial components of drug development, ensuring therapeutic efficacy and improving bioavailability. Nanoparticle drug carriers have gained significant attention for their role in drug product stability and dosage form design. Advancements in pulmonary and inhalation drug delivery systems have led to improvements in drug metabolism and elimination pathways, while liposomal drug delivery systems have shown promise in enhancing drug solubility and stability. Targeted drug release and sustained release technology continue to dominate the market, with biodegradable polymers and controlled release formulations driving growth. Pharmaceutical nanotechnology has emerged as a key trend, offering significant potential for drug delivery mechanisms and bioavailability improvement.
- The market is characterized by continuous innovation and competition, with companies focusing on dosing regimen design and patient compliance improvement. The drug delivery mechanisms landscape is diverse, with ongoing research and development efforts in various areas, including drug metabolism, toxicokinetics studies, and pharmaceutical nanotechnology.
What are the Key Data Covered in this New Drug Delivery Systems Market Research and Growth Report?
-
What is the expected growth of the New Drug Delivery Systems Market between 2025 and 2029?
-
USD 59.4 billion, at a CAGR of 4.6%
-
-
What segmentation does the market report cover?
-
The report segmented by Application (Oncology, Neurology, Cardiology, Diabetes, and Others), Route Of Administration (Oral drug delivery systems, Injectable drug delivery systems, Pulmonary drug delivery systems, Transdermal drug delivery systems, and Others), Method (Targeted drug delivery systems, Controlled drug delivery systems, and Modulated drug delivery systems), and Geography (North America, Europe, Asia, and Rest of World (ROW))
-
-
Which regions are analyzed in the report?
-
North America, Europe, Asia, and Rest of World (ROW)
-
-
What are the key growth drivers and market challenges?
-
Rising prevalence of chronic diseases, Stringent regulations
-
-
Who are the major players in the New Drug Delivery Systems Market?
-
Key Companies Abbott Laboratories, Amgen Inc., Antares Pharma Inc., AstraZeneca Plc, Baxter International Inc., Becton Dickinson and Co., Boehringer Ingelheim International GmbH, Eli Lilly and Co., F. Hoffmann La Roche Ltd., Gerresheimer AG, Gilead Sciences Inc., Kindeva Drug Delivery L.P., Medtronic Plc, Merck KGaA, Nemera Group, Pfizer Inc., Sanofi SA, Teva Pharmaceutical Industries Ltd., West Pharmaceutical Services Inc., and Ypsomed Holding AG
-
Market Research Insights
- The market encompasses a diverse range of technologies, including swellable polymers and erodible polymers, controlled release matrix systems, and protein and gene delivery systems. These advanced systems offer enhanced absorption kinetics and improved drug targeting, leading to increased efficacy and reduced side effects. For instance, implant delivery systems have shown a 30% increase in therapeutic efficacy compared to traditional oral administration. Moreover, the market incorporates various manufacturing processes, such as solid dispersions and polymeric nanoparticles, as well as lipid-based nanoparticles, to ensure excipient compatibility and meet regulatory guidelines. Quality control measures, including stability testing methods and process validation, are crucial for ensuring the safety and efficacy of these complex drug delivery systems.
- In the realm of vaccine delivery, the market has witnessed significant advancements, with drug infusion pumps and inhaler devices enabling precise dosing and enhanced patient compliance. Peptide delivery systems and receptor-mediated drug delivery have shown promise in improving drug permeability and drug safety assessment. The drug product lifecycle involves extensive research and development, including drug interaction studies, clinical pharmacology, and patient outcome measures, to ensure optimal performance and regulatory approval. Biocompatible materials and syringe pumps are essential components in the manufacturing of these sophisticated drug delivery systems.
We can help! Our analysts can customize this new drug delivery systems market research report to meet your requirements.