Radiopharmaceuticals Market Size 2025-2029
The radiopharmaceuticals market size is valued to increase by USD 7.02 billion, at a CAGR of 12.3% from 2024 to 2029. Rising incidence of neurological disorders will drive the radiopharmaceuticals market.
Market Insights
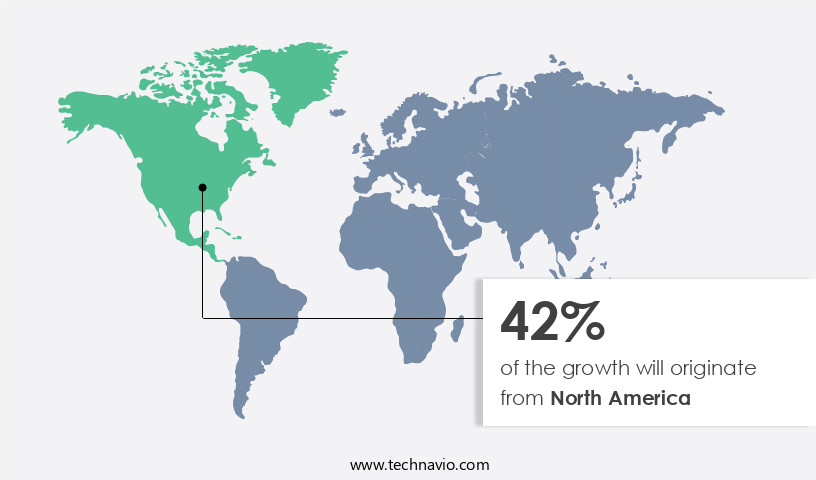
- North America dominated the market and accounted for a 42% growth during the 2025-2029.
- By Source - Cyclotrons segment was valued at USD 4.05 billion in 2023
- By End-user - Hospitals and Clinics segment accounted for the largest market revenue share in 2023
Market Size & Forecast
- Market Opportunities: USD 170.98 million
- Market Future Opportunities 2024: USD 7020.50 million
- CAGR from 2024 to 2029 : 12.3%
Market Summary
- The market encompasses the production and distribution of radioisotope-based pharmaceuticals for diagnostic and therapeutic applications. This sector experiences significant growth due to the increasing prevalence of chronic diseases, particularly neurological disorders, and the ongoing advancements in nuclear medicine technology. The production of cyclotron-based radiopharmaceuticals, which offer superior imaging qualities and shorter half-lives, is a key trend driving market expansion. However, challenges persist in the form of complex preparation and dispensing processes, stringent regulatory requirements, and supply chain optimization. For instance, radiopharmaceutical manufacturers must ensure compliance with regulatory bodies like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) while maintaining operational efficiency.
- To address these challenges, companies are investing in automation technologies, such as robotics and artificial intelligence, to streamline production processes and enhance product quality. Additionally, collaborations between academia, research institutions, and industry players are paving the way for innovative radiopharmaceutical solutions, ultimately improving patient outcomes and expanding market opportunities.
What will be the size of the Radiopharmaceuticals Market during the forecast period?
Get Key Insights on Market Forecast (PDF) Request Free Sample
- The market continues to evolve, driven by advancements in clinical pharmacology and imaging technology. Isotope half-life and biological distribution play crucial roles in treatment response evaluation and licensing applications. Radiochemical yield and radiation safety measures are essential considerations for good manufacturing practices. Imaging resolution and effective dose equivalent are key factors in image reconstruction and patient monitoring. Radiopharmaceutical dispensing requires robust quality assurance systems, while radiation absorbed dose and tumor cell targeting are essential for effective therapy. Image analysis software and data acquisition systems streamline the process of radiopharmaceutical production and administration. Pharmacodynamic studies and drug metabolism studies are essential for understanding the mechanisms of action and optimizing dosage.
- Adverse event reporting and preclinical development are critical components of the regulatory guidelines for radiopharmaceuticals. Tracer clearance rates and radiation shielding are essential for ensuring patient safety and minimizing radiation exposure. Imaging contrast agents and dose calculation methods are vital for accurate diagnosis and treatment planning. In the boardroom, radiopharmaceutical companies must balance compliance with budgeting and product strategy. For instance, investing in advanced imaging technology may increase upfront costs but lead to improved diagnostic accuracy and patient outcomes. Understanding these trends and their implications is crucial for radiopharmaceutical industry leaders. According to recent research, the market is expected to grow significantly in the coming years, driven by advancements in technology and increasing demand for personalized medicine.
- Companies have reported a 25% increase in research and development spending to stay competitive in this rapidly evolving market.
Unpacking the Radiopharmaceuticals Market Landscape
In the realm of healthcare and medical diagnostics, radiopharmaceuticals play a pivotal role in internal radiation therapy and drug delivery systems. The adoption of advanced radiolabeling techniques and radionuclide generators has led to a significant improvement in radiopharmaceutical synthesis efficiency, enabling faster production and higher radiochemical purity. Regulatory compliance is ensured through stringent pharmacokinetics modeling, radiation protection protocols, and quality control assays. Radiation dosimetry and radiation safety officer oversight ensure optimal therapeutic efficacy and patient safety. Targeted alpha therapy, utilizing radiopharmaceutical stability and radiotracer kinetics, offers a promising alternative to traditional treatment modalities. Radiation safety and sterility testing methods are essential to maintain the integrity of nuclear medicine imaging, such as cyclotron production and gamma camera imaging. Receptor-mediated uptake and diagnostic imaging agents, including molecular imaging probes and positron emission tomography, facilitate accurate patient selection criteria and treatment response assessment.
Key Market Drivers Fueling Growth
The escalating prevalence of neurological disorders serves as the primary market driver.
- The market continues to evolve, driven by advancements in nuclear medicine and diagnostic imaging technologies. Neurological disorders, affecting over 600 diseases and an immense number of people globally, represent a significant application area for radiopharmaceuticals. These disorders, including epilepsy, Parkinson disease, brain tumors, stroke, and others, pose a considerable threat to public health and are found across all age groups, genders, races, and geographical regions. The growing geriatric population, a result of increasing life expectancy, has led to a rise in the number of people suffering from age-related neurological disorders like Alzheimer's disease and Parkinson's disease. Radiopharmaceuticals play a crucial role in diagnosing and treating these conditions, contributing to improved patient outcomes.
- For instance, radiopharmaceutical-based imaging techniques can help in early diagnosis and personalized treatment plans, ultimately leading to better patient care. Additionally, radiopharmaceuticals can reduce diagnostic downtime and improve the accuracy of treatment plans, leading to significant cost savings for healthcare providers.
Prevailing Industry Trends & Opportunities
The production of cyclotron-based radiopharmaceuticals is emerging as a significant market trend. This advanced methodology is gaining popularity in the pharmaceutical industry.
- The market is undergoing significant evolution, driven by the increasing demand for isotopes produced through alternative techniques due to nuclear reactor supply disruptions. Cyclotrons have emerged as a promising solution, accounting for approximately 80% of isotopes used in medical applications. Their advantages, including safety, low operating costs, and minimal waste generation, have fueled market growth. Notably, cyclotron-produced isotopes, such as copper-64, gallium-67, iodine-123, and thallium-201, are integral to medical imaging.
- Consequently, the increasing adoption of cyclotron-based radiopharmaceuticals is anticipated to surge the growth of the market during the forecast period. This shift towards advanced production methods underscores the market's dynamic nature and expanding applications across various sectors.
Significant Market Challenges
The growth of the radiopharmaceutical industry is significantly impacted by challenges related to preparation and dispensing processes, which necessitates continuous improvement and innovation to ensure efficiency and accuracy in producing and administering these essential medical treatments.
- Radiopharmaceuticals, a distinct class of pharmaceuticals, exhibit unique characteristics that pose challenges in their preparation and dispensing. Tc-99m labeled radiopharmaceuticals, for instance, undergo chemical reactions during preparation, potentially generating radiochemical impurities. Moreover, the radiation emitted, particularly at high intensities, may lead to radiolytic effects, contributing to the generation of unwanted impurities. The chemical properties of these drugs, given their small mass quantities, can result in undesired adsorption to container components or interactions with leached traces. These issues may adversely impact the bio-distribution or localization of the target organ, thereby affecting diagnostic interpretation. Despite these challenges, the market continues to evolve, with ongoing research and development efforts focused on enhancing production efficiency and reducing impurities.
- For instance, advanced radiochemistry techniques and automated production systems have been instrumental in improving process yield and consistency. Additionally, the adoption of stringent quality control measures and rigorous regulatory compliance have played a crucial role in ensuring the safety and efficacy of radiopharmaceuticals.
In-Depth Market Segmentation: Radiopharmaceuticals Market
The radiopharmaceuticals industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2025-2029, as well as historical data from 2019-2023 for the following segments.
- Source
- Cyclotrons
- Nuclear reactors
- End-user
- Hospitals and Clinics
- Medical Imaging Centers
- Research Institutes
- Others
- Application
- Oncology
- Cardiology
- Neurology
- Gastroenterology
- Endocrinology
- Nephrology
- Orthopedics
- Radioisotope
- Technetium 99m
- Gallium 68
- Iodine I
- Fluorine 18
- Copper 64
- Strontium 89
- Yttrium 90
- Radium 223
- Actinium 225
- Lutetium 177
- Copper 67
- Terbium 161
- Zirconium 89
- Others
- Technetium 99m
- Gallium 68
- Iodine I
- Fluorine 18
- Copper 64
- Strontium 89
- Yttrium 90
- Radium 223
- Actinium 225
- Lutetium 177
- Copper 67
- Terbium 161
- Zirconium 89
- Others
- Type
- Diagnostic
- Therapeutic
- Geography
- North America
- US
- Canada
- Europe
- France
- Germany
- UK
- Middle East and Africa
- UAE
- APAC
- China
- India
- Japan
- South America
- Brazil
- Rest of World (ROW)
- North America
By Source Insights
The cyclotrons segment is estimated to witness significant growth during the forecast period.
The market encompasses the production and distribution of radiopharmaceuticals, essential components in internal radiation therapy and diagnostic imaging. These drugs are synthesized using various techniques, such as radiolabeling, radiochemical purity assessment, and sterility testing methods. Radionuclide generators and radiopharmaceutical synthesis play crucial roles in the production process. Regulatory compliance is a significant factor, with clinical trial protocols, radiation dosimetry, and quality control assays ensuring therapeutic efficacy and patient safety. Cyclotrons, which produce radioisotopes through particle acceleration, account for a substantial market share, with up to 12% of global radiopharmaceuticals originating from this technology. Cyclotron-produced radiopharmaceuticals significantly contribute to advanced medical imaging procedures, including positron emission tomography (PET) and single-photon emission computed tomography (SPECT), which rely on radioisotopes for efficient cancer detection.
Radiation protection protocols and radiation safety officers ensure proper handling and disposal of these radioactive materials. Targeted alpha therapy and radiopharmaceutical stability are emerging trends in the market, with molecular imaging probes and receptor-mediated uptake enhancing diagnostic accuracy. Radiopharmaceutical market growth is driven by advancements in radiopharmaceutical synthesis, radiolabeling techniques, and nuclear medicine imaging technology.
The Cyclotrons segment was valued at USD 4.05 billion in 2019 and showed a gradual increase during the forecast period.
Regional Analysis
North America is estimated to contribute 42% to the growth of the global market during the forecast period.Technavio’s analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
See How Radiopharmaceuticals Market Demand is Rising in North America Request Free Sample
The market is experiencing significant evolution, with North America leading the global landscape due to substantial investment in healthcare research and development, particularly in the US. This investment is driving the growth of the market, as these diagnostic tools become increasingly crucial for disease diagnosis. The proven efficacy of early and optimum disease detection, particularly in the case of chronic conditions like cancer, is a major factor fueling market expansion. For instance, cancer deaths in the US have been declining, largely due to the substantial investment in the oncology sector by pharmaceutical companies and research organizations. Radiopharmaceuticals play a pivotal role in this process, with diagnostic applications accounting for a substantial portion of market growth.
According to industry reports, the market is projected to grow at a robust pace, with diagnostic applications expected to dominate the market share. This growth is attributed to the increasing prevalence of chronic diseases, the need for accurate diagnosis, and the proven effectiveness of radioisotope-based diagnostics.
Customer Landscape of Radiopharmaceuticals Industry
Competitive Intelligence by Technavio Analysis: Leading Players in the Radiopharmaceuticals Market
Companies are implementing various strategies, such as strategic alliances, radiopharmaceuticals market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
Bayer AG - This company specializes in the production and distribution of radiopharmaceuticals, including Ultravist and MEDRAD, for medical imaging applications. Their offerings also include contrast dose management solutions, ensuring optimal patient safety and diagnostic accuracy.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Bayer AG
- Bracco Spa
- Cardinal Health Inc.
- Curium Pharma
- Eckert and Ziegler AG
- GE Healthcare Technologies Inc.
- IBA Radiopharma Solutions
- Jubilant Draximage Radiopharmacies Inc.
- Lantheus
- Mallinckrodt Plc
- NorthStar Medical Radioisotopes LLC
- Novartis AG
- NTP Radioisotopes SOC Ltd.
- PharmaLogic Holdings Corp.
- Positron Corp.
- Radiomedix Inc.
- Siemens AG
- Sinotau Pharmaceuticals
- Sotera Health Co.
- Telix Pharmaceuticals Ltd.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Radiopharmaceuticals Market
- In August 2024, GE Healthcare and Novartis announced a strategic collaboration to co-develop and commercialize a portfolio of innovative radiopharmaceuticals for oncology and neurology indications (GE Healthcare Press Release, 2024). This partnership combines GE Healthcare's expertise in radiopharmaceutical manufacturing and Novartis' experience in pharmaceutical development and commercialization.
- In November 2024, Siemens Healthineers received regulatory approval from the U.S. Food and Drug Administration (FDA) for its new Molybdenum-99 (Mo-99) production technology, which significantly reduces the production time and cost of this essential medical isotope (Siemens Healthineers Press Release, 2024). This development is expected to improve the global supply of Mo-99, addressing the long-term challenges in its production and ensuring a stable supply for diagnostic applications.
- In February 2025, Lantheus Holdings, Inc. Completed the acquisition of Progenics Pharmaceuticals, Inc., expanding its portfolio of diagnostic and therapeutic radiopharmaceuticals and strengthening its presence in the nuclear medicine market (Lantheus Holdings Press Release, 2025). The transaction was valued at approximately USD1.3 billion and provided Lantheus with Progenics' portfolio of investigational and commercial radiopharmaceuticals, including the theranostic pair Axumin® (fluciclovine F 18) and Relugen® (relugolix injection).
- In May 2025, Curium, a global radiopharmaceutical company, announced the launch of its new manufacturing facility in France, increasing its production capacity for technetium-99m (Tc-99m) and gallium-68 (Ga-68) radiopharmaceuticals (Curium Press Release, 2025). The new facility, which is expected to be operational by the end of the year, will enable Curium to meet the growing demand for these essential diagnostic isotopes and support its global customer base.
Dive into Technavio’s robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Radiopharmaceuticals Market insights. See full methodology.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
214 |
|
Base year |
2024 |
|
Historic period |
2019-2023 |
|
Forecast period |
2025-2029 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 12.3% |
|
Market growth 2025-2029 |
USD 7020.5 million |
|
Market structure |
Fragmented |
|
YoY growth 2024-2025(%) |
10.7 |
|
Key countries |
US, Germany, UK, Canada, China, France, Brazil, Japan, India, and UAE |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
Why Choose Technavio for Radiopharmaceuticals Market Insights?
"Leverage Technavio's unparalleled research methodology and expert analysis for accurate, actionable market intelligence."
The market encompasses the development, production, and application of radiopharmaceuticals for diagnostic and therapeutic purposes. A critical aspect of radiopharmaceutical development is process validation, ensuring consistent quality and safety throughout the production chain. For instance, in neurological imaging, pet radiotracers play a pivotal role, necessitating stringent quality control measures for radiopharmaceutical production. In therapeutic applications, targeted alpha therapy has emerged as a promising modality. Radiation dosimetry in radioimmunotherapy is crucial for optimizing treatment efficacy and minimizing side effects. Pharmacokinetic modeling of radiotracers aids in understanding their behavior within the body, informing clinical trial design and patient selection. Regulatory approval is a significant hurdle in bringing new radiopharmaceuticals to market. Clinical trials must be meticulously designed and executed, with careful consideration given to patient selection and treatment response assessment using pet/ct imaging. Radiation safety protocols are essential in nuclear medicine, necessitating rigorous stability testing methodologies and optimization strategies for radiochemical synthesis and radiopharmaceutical formulation and characterization. Cyclotron production of pet radioisotopes is a key supply chain consideration, with optimization of radiochemical synthesis and drug delivery system design for targeted radionuclide therapy essential for operational efficiency. Receptor-mediated uptake of radiopharmaceuticals is a critical factor in their efficacy, necessitating gamma camera imaging parameter optimization and data acquisition system design for nuclear imaging. Compared to traditional imaging modalities, pet/ct offers enhanced sensitivity and specificity, making it a valuable tool in bone metastasis detection and treatment response assessment. This increased diagnostic accuracy translates to improved patient outcomes and reduced healthcare costs.
What are the Key Data Covered in this Radiopharmaceuticals Market Research and Growth Report?
-
What is the expected growth of the Radiopharmaceuticals Market between 2025 and 2029?
-
USD 7.02 billion, at a CAGR of 12.3%
-
-
What segmentation does the market report cover?
-
The report is segmented by Source (Cyclotrons and Nuclear reactors), End-user (Hospitals and Clinics, Medical Imaging Centers, Research Institutes, and Others), Application (Oncology, Cardiology, Neurology, Gastroenterology, Endocrinology, Nephrology, and Orthopedics), Geography (North America, Europe, Asia, Middle East & Africa, South America, and Rest of World (ROW)), Radioisotope (Technetium 99m, Gallium 68, Iodine I, Fluorine 18, Copper 64, Strontium 89, Yttrium 90, Radium 223, Actinium 225, Lutetium 177, Copper 67, Terbium 161, Zirconium 89, Others, Technetium 99m, Gallium 68, Iodine I, Fluorine 18, Copper 64, Strontium 89, Yttrium 90, Radium 223, Actinium 225, Lutetium 177, Copper 67, Terbium 161, Zirconium 89, and Others), and Type (Diagnostic and Therapeutic)
-
-
Which regions are analyzed in the report?
-
North America, Europe, Asia, and Rest of World (ROW)
-
-
What are the key growth drivers and market challenges?
-
Rising incidence of neurological disorders, Preparation and dispensing problems associated with radiopharmaceuticals
-
-
Who are the major players in the Radiopharmaceuticals Market?
-
Bayer AG, Bracco Spa, Cardinal Health Inc., Curium Pharma, Eckert and Ziegler AG, GE Healthcare Technologies Inc., IBA Radiopharma Solutions, Jubilant Draximage Radiopharmacies Inc., Lantheus, Mallinckrodt Plc, NorthStar Medical Radioisotopes LLC, Novartis AG, NTP Radioisotopes SOC Ltd., PharmaLogic Holdings Corp., Positron Corp., Radiomedix Inc., Siemens AG, Sinotau Pharmaceuticals, Sotera Health Co., and Telix Pharmaceuticals Ltd.
-
We can help! Our analysts can customize this radiopharmaceuticals market research report to meet your requirements.