Celiac Diseases Drugs Market Size 2025-2029
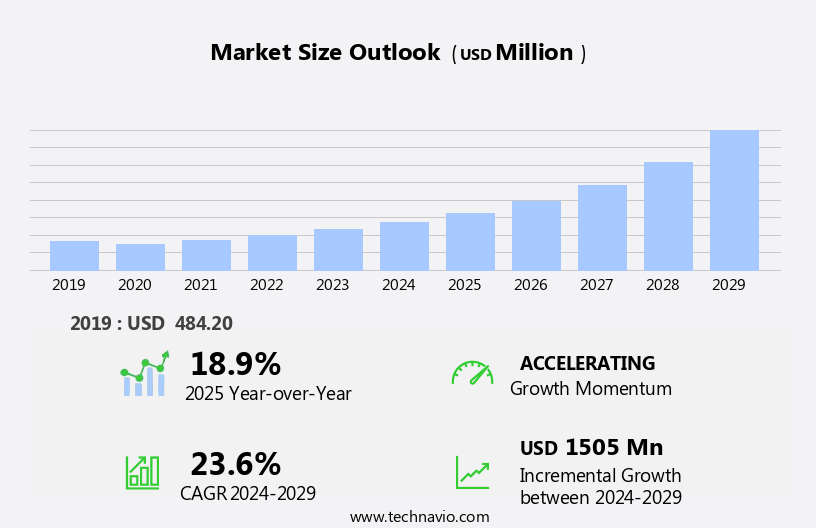
The celiac diseases drugs market size is forecast to increase by USD 1.51 billion, at a CAGR of 23.6% between 2024 and 2029.
- The market is driven by the increasing consumption of gluten-containing food, which leads to a higher prevalence of celiac disease and subsequent demand for effective treatment options. The market is also expected to benefit from advancements in drug formulation technology and healthcare systems, which will improve the accessibility and affordability of treatments for patients. Despite these growth factors, the market faces hurdles, including the high cost of treatments and the limited availability of insurance coverage for celiac disease-related drugs. The market also faces challenges in the area of diagnosis. Despite the availability of diagnostic tests, many individuals with celiac disease remain undiagnosed, leading to potential long-term health complications. Furthermore, inorganic growth strategies, such as acquisitions and collaborations, are being employed by market players to expand their product portfolios and cater to the growing patient population. These strategies enable companies to offer a broader range of treatments and improve patient outcomes, making them key differentiators in the competitive landscape.
- Effective management of these challenges, particularly in the realm of diagnosis, will be crucial for market success. Companies that can streamline the diagnostic process and ensure early detection will gain a competitive edge, ultimately improving patient quality of life and driving market growth.
What will be the Size of the Celiac Diseases Drugs Market during the forecast period?
Explore in-depth regional segment analysis with market size data - historical 2019-2023 and forecasts 2025-2029 - in the full report.
Request Free Sample
The celiac diseases market continues to evolve, shaped by the ongoing unfolding of market dynamics and applications across various sectors. Endoscopic procedures remain a crucial diagnostic tool, while malabsorption syndromes drive the need for innovative treatments. Intellectual property rights and healthcare costs are key considerations in the development of new therapeutic options. Expert consensus and health technology assessment play a significant role in treatment guidelines, emphasizing the importance of patient education and adherence to gluten-free diets. Longitudinal studies and disease awareness campaigns contribute to a better understanding of disease progression and the impact of immunomodulatory therapies on health outcomes.
Small intestinal biopsies and genetic testing provide valuable insights into disease epidemiology and the role of HLA-DQ2/DQ8 in celiac disease. Healthcare policies and reimbursement strategies influence market access, while economic modeling and cost-effectiveness analysis inform decision-making in celiac disease management. Drug development for celiac disease is ongoing, with a focus on enzyme replacement therapy, dietary supplements, and novel immunomodulatory agents. Autoimmune diseases and nutritional deficiencies associated with celiac disease further expand the therapeutic landscape. Clinical trials and regulatory pathways are essential components of drug approval, ensuring safety and efficacy for patients. The global healthcare trends towards personalized medicine and patient-centered care continue to impact the celiac diseases market, with a growing emphasis on disease remission and symptom management.
The role of gluten-free food products and food labeling regulations in celiac disease diagnosis and treatment is an evolving area of research and policy development. Market access and disease epidemiology are interconnected, with research funding and public health initiatives playing a critical role in addressing the unmet needs of the celiac disease community. The ongoing dynamic nature of the celiac diseases market underscores the importance of continued investment in research, education, and patient support.
How is this Celiac Diseases Drugs Industry segmented?
The celiac diseases drugs industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2025-2029, as well as historical data from 2019-2023 for the following segments.
- Therapy
- First line treatment
- Second-line treatment
- Route Of Administration
- Oral
- Parenteral
- Distribution Channel
- Hospitals and specialty clinics
- Specialty pharmacies
- Retail pharmacies
- Online platforms
- Geography
- North America
- US
- Canada
- Europe
- France
- Germany
- Italy
- The Netherlands
- UK
- APAC
- China
- India
- Japan
- Rest of World (ROW)
- North America
By Therapy Insights
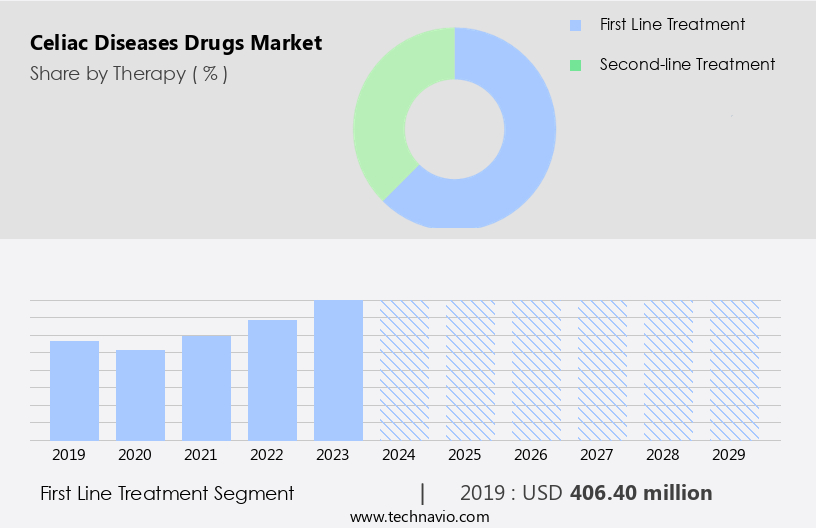
The first line treatment segment is estimated to witness significant growth during the forecast period.
The celiac disease market encompasses a range of therapeutic options, with the primary focus on prevention and managing the disease. Currently, a gluten-free diet serves as the first line of treatment, aiming to prevent immune responses and associated clinical manifestations. This dietary approach is expected to witness significant growth due to ongoing research and development. Several drugs are under fast-track approval processes, set to enter the market in the forecast period. Notably, there are currently no approved drugs for celiac disease management, making the impact of these upcoming approvals substantial. Health literacy and patient education play a crucial role in disease awareness and treatment adherence.
Treatment guidelines and health technology assessment are essential for effective disease management and cost-effective strategies. Immunomodulatory therapies and enzyme replacement therapies are among the emerging treatment options, offering potential health outcomes for patients. Small intestinal biopsies and genetic testing are integral to accurate diagnosis, while healthcare policies and reimbursement strategies influence market access. The global healthcare trends, including an increasing prevalence of celiac disease and autoimmune diseases, further fuel market growth. However, medication side effects and disease progression necessitate continuous research and development to address nutritional deficiencies and malabsorption syndromes. Expert consensus, cost-effectiveness analysis, and economic modeling contribute to drug approval and market access.
Drug safety, clinical trials, and regulatory pathways are essential components of drug development, ensuring drug efficacy and patient compliance. Public health initiatives and research funding are vital for disease epidemiology and disease awareness campaigns. Longitudinal studies and scientific publications provide valuable insights into disease progression and treatment guidelines. Ultimately, the market's evolution reflects the ongoing commitment to improving celiac disease management and patient outcomes.
The First line treatment segment was valued at USD 406.40 billion in 2019 and showed a gradual increase during the forecast period.
Regional Analysis
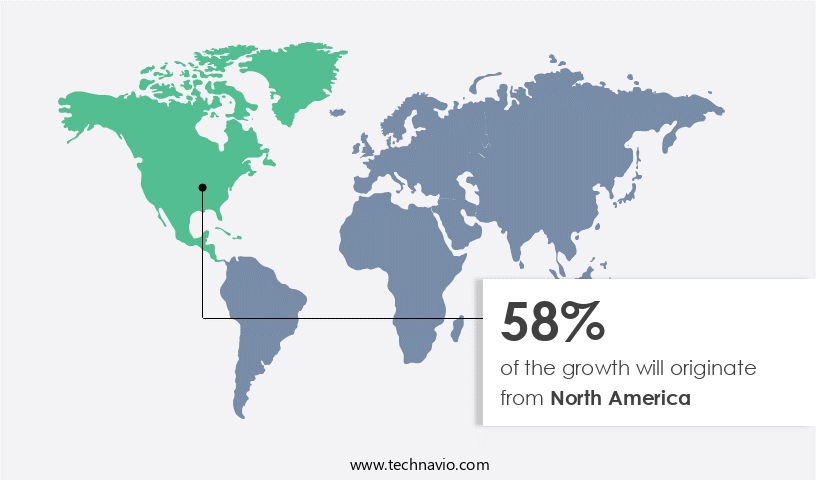
North America is estimated to contribute 58% to the growth of the global market during the forecast period. Technavio's analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
The celiac diseases market is driven by the rising prevalence of celiac diseases, growing health literacy, and increasing awareness campaigns. In the US, the disease affects over 3 million people, with the number increasing due to the consumption of gluten-containing foods, particularly beer and fast food. Treatment options include gluten-free diets, immunomodulatory therapies, and enzyme replacement therapy. Patient education and adherence to treatment guidelines are crucial for effective disease management. Health technology assessment plays a significant role in evaluating treatment options and their cost-effectiveness. Healthcare policies and reimbursement strategies also influence market dynamics. Medication side effects and healthcare costs are key concerns.
Global healthcare trends towards personalized medicine and genetic testing are shaping the market. Disease progression and nutritional deficiencies necessitate ongoing research and clinical trials. Autoimmune diseases, such as celiac disease, are a growing focus in the pharmaceutical industry. Market access and regulatory pathways are essential for drug approval and commercialization. Expert consensus and scientific publications contribute to the development of treatment guidelines and best practices. Economic modeling and disease remission studies help assess the long-term impact of various treatments. The market for celiac disease drugs is expected to grow due to the increasing prevalence of the disease, the development of new treatments, and the growing awareness of the disease and its impact on quality of life.
The market for celiac disease drugs is diverse, with various therapeutic options, including gluten-free food products, enzyme replacement therapy, and immunomodulatory therapies. Patient compliance and symptom management are key challenges in the market. Small intestinal biopsies and endoscopic procedures are used for diagnosis, while drug safety and food labeling regulations ensure the safety and efficacy of treatments. The market for celiac disease drugs is expected to grow significantly due to the increasing prevalence of the disease, the development of new treatments, and the growing awareness of the disease and its impact on quality of life.
Market Dynamics
Our researchers analyzed the data with 2024 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
The market represents a significant and growing sector within the pharmaceutical industry, focusing on the development and commercialization of treatments for celiac disease, an autoimmune disorder triggered by the ingestion of gluten. This condition damages the small intestine, leading to nutrient malabsorption and various symptoms. Key players in this market are actively researching and innovating to address the unmet needs of the celiac population, including those who do not respond to a gluten-free diet. Drugs under development include enzyme therapies, immunomodulators, and vaccines. Additionally, there is a growing focus on improving patient compliance, diagnostics, and early intervention to mitigate long-term health complications. The market is driven by increasing awareness, diagnosis rates, and the expanding global prevalence of celiac disease.
What are the key market drivers leading to the rise in the adoption of Celiac Diseases Drugs Industry?
- The significant consumption of gluten-containing foods serves as the primary market driver.
- Celiac disease is a gastrointestinal disorder characterized by an adverse reaction to gluten, a protein found in wheat, barley, and rye. The prevalence of this condition is on the rise due to increased consumption of foods containing gluten, such as pizza, cake, soups, beer, bread, cookies, pasta, and burgers. Longitudinal studies suggest that HLA-DQ2/DQ8 genes increase the risk of developing celiac disease. Healthcare policies and reimbursement strategies play a crucial role in celiac disease management, which includes medication and dietary supplements.
- Enzyme replacement therapy and dietary modifications are common treatment approaches. However, medication side effects and adverse drug reactions are concerns for patients. Global healthcare trends indicate a growing focus on patient compliance and personalized care in managing chronic conditions like celiac disease. Understanding the disease and its implications is essential for effective management and improving the quality of life for affected individuals.
What are the market trends shaping the Celiac Diseases Drugs Industry?
- Inorganic growth strategies, such as mergers and acquisitions or strategic partnerships, are currently gaining traction in the business world as effective methods for expanding a company's reach and market share. These strategies allow organizations to grow more rapidly than through organic means alone, enabling them to compete more effectively and seize new opportunities in the market.
- The market is driven by several factors, including the increasing prevalence of malabsorption syndromes and autoimmune diseases. According to expert consensus, celiac disease affects approximately 1 in 100 people worldwide, leading to nutritional deficiencies and endoscopic procedures. The high healthcare costs associated with managing these conditions create a significant market opportunity for new treatments. Intellectually-protected drugs with proven cost-effectiveness are in high demand. Market access is a critical consideration for both patients and healthcare providers, making clinical trials and drug development essential. Genetic testing plays a crucial role in diagnosing celiac disease, and ongoing research aims to improve diagnostic accuracy.
- Inorganic growth strategies, such as mergers and acquisitions, partnerships, and regional acquisitions, are expected to contribute to market expansion. For instance, Takeda and Zedira's collaboration and licensing agreement for the development of ZED1227/TAK-227, a Phase 2b investigational therapy for celiac disease, underscores the importance of these strategies. The market's future consolidation is expected to accelerate as a result. Disease epidemiology, intellectual property, and cost-effectiveness analysis are essential factors influencing the market's growth trajectory. The market's ongoing evolution reflects the industry's commitment to addressing the unmet needs of patients and healthcare providers.
What challenges does the Celiac Diseases Drugs Industry face during its growth?
- A major industry challenge is the concern over inadequate diagnoses, which can hinder growth.
- Celiac disease, an autoimmune disorder, presents challenges in diagnosis due to its non-specific symptoms and the lack of a definitive biomarker. Consequently, many individuals remain undiagnosed, with estimates suggesting that up to eight out of ten cases go unidentified. The disease can manifest at any age, with the average diagnosis occurring between the ages of 30 and 50. The complexities of celiac disease necessitate extensive research and public health initiatives to improve diagnosis and treatment. Regulatory pathways for drug approval are crucial in addressing the unmet medical needs of celiac disease patients. Economic modeling and scientific publications contribute significantly to the understanding of disease remission and drug efficacy.
- Drug safety and symptom management are essential considerations in the development of new treatments. Gluten-free food products and food labeling regulations play a vital role in managing celiac disease, but they do not replace the need for effective pharmaceutical interventions. The importance of rigorous clinical trials and robust data analysis cannot be overstated in the quest for drug approval. Disease remission is the ultimate goal in celiac disease treatment, and ongoing research aims to develop drugs that can achieve this objective. The scientific community continues to publish research on celiac disease, shedding light on its complexities and potential treatment options.
- In conclusion, the celiac disease market dynamics revolve around the need for accurate diagnosis, effective drug development, and improved disease management. The challenges associated with celiac disease necessitate a multifaceted approach that incorporates research funding, public health initiatives, regulatory pathways, and scientific advancements.
Exclusive Customer Landscape
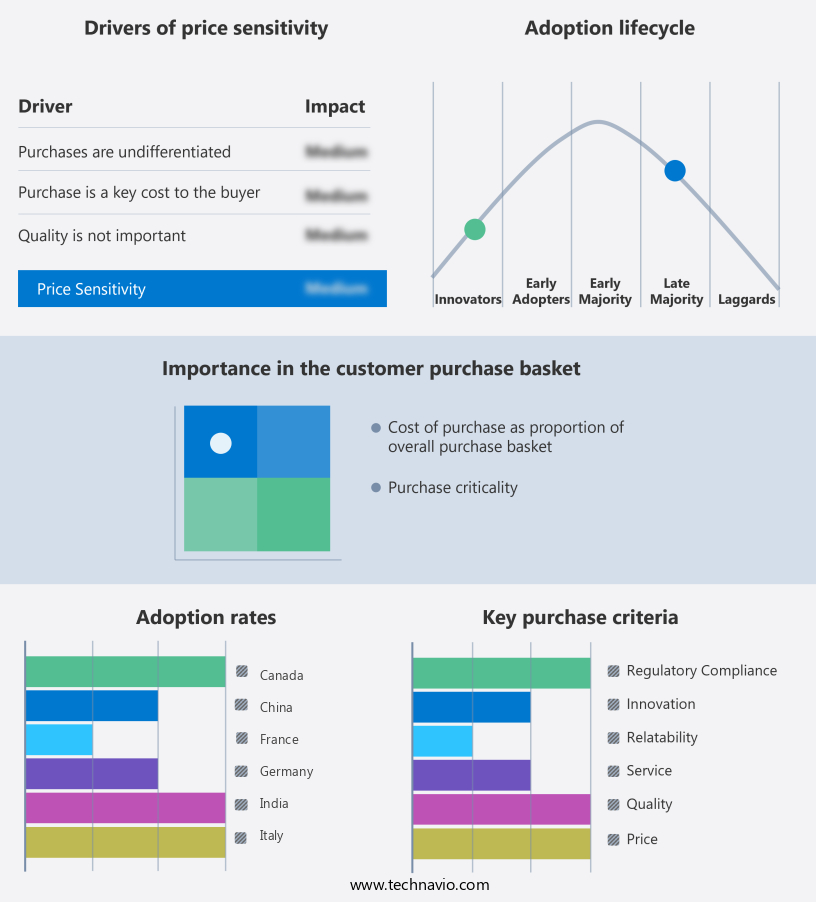
The celiac diseases drugs market forecasting report includes the adoption lifecycle of the market, covering from the innovator's stage to the laggard's stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the celiac diseases drugs market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape
Key Companies & Market Insights
Companies are implementing various strategies, such as strategic alliances, celiac diseases drugs market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
9 Meters Biopharma Inc - This company specializes in innovative drug solutions for celiac diseases, including Immunoseq dx, Pairseq, and Trutcr.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- 9 Meters Biopharma Inc
- Adaptive Biotechnologies Corp.
- Almirall SA
- Amgen Inc.
- AMYRA Biotech AG
- Artielle Immunotherapeutics Inc
- Avaxia Biologics Inc.
- BioLineRx Ltd.
- Bristol Myers Squibb Co.
- Calypso Biotech BV
- GlaxoSmithKline Plc
- Glenmark Pharmaceuticals Ltd.
- ImmunogenX Inc.
- Johnson and Johnson Services Inc.
- Precigen Inc.
- Sanofi SA
- Takeda Pharmaceutical Co. Ltd.
- Teva Pharmaceutical Industries Ltd.
- Vactech Oy
- Zedira GmbH
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Celiac Diseases Drugs Market
- In January 2024, Biomarca, a biotech company specializing in celiac disease diagnostics, announced the successful completion of a USD20 million Series B funding round. Investors included new backers OrbiMed and Sofinnova Ventures, as well as existing investors 5AM Ventures and Frazier Healthcare Partners (Source: BusinessWire).
- In March 2024, Alvine Pharmaceuticals, a clinical-stage biotech company, reported positive top-line results from a Phase 2b trial of ALV003, its investigational gluten tolerance inducer for celiac disease. The trial met its primary and secondary endpoints, demonstrating a significant reduction in intestinal mucosal damage (Source: GlobeNewswire).
- In May 2024, Nestle Health Science and Celiac Disease Foundation announced a strategic collaboration to develop new celiac disease care solutions. The partnership aims to improve diagnosis, management, and education for celiac disease patients (Source: PR Newswire).
- In April 2025, the U.S. Food and Drug Administration (FDA) granted Orphan Drug Designation to ImmusanT's lead investigational therapy, Nexvax2, for the prevention of celiac disease in genetically susceptible individuals. This designation provides the company with various development incentives, including tax credits and seven years of market exclusivity upon approval (Source: BusinessWire).
Research Analyst Overview
- The celiac diseases market is characterized by a growing focus on nutritional support and patient advocacy to improve the quality of life for those diagnosed with celiac disease and related conditions. Nutritional assessment plays a crucial role in managing the disease, particularly in addressing iron deficiency anemia and other nutrient deficiencies. Therapeutic efficacy of medication therapy is under constant scrutiny, with ongoing research into the role of immunosuppressive drugs and behavioral interventions. Dietary management remains a cornerstone of treatment, with a shift towards incorporating more trace elements, dietary fiber, and lifestyle modifications. Environmental factors and neurological manifestations continue to pose challenges in disease management, necessitating epidemiological surveillance and adverse event monitoring.
- Dermatitis herpetiformis, a related condition, also requires careful management. Weight loss and abdominal pain are common symptoms, further emphasizing the need for effective health promotion and disease prevention strategies. Genetic predisposition and non-celiac gluten sensitivity are emerging areas of interest, with mental health and early detection also becoming increasingly important. Data analytics and support groups are valuable resources for patients, providing valuable insights into disease management and facilitating communication among those affected. Adverse event monitoring and lifestyle modifications are essential components of care, with medication therapy playing a supportive role. Risk factors, including wheat allergy and neurological manifestations, require ongoing monitoring and management to optimize patient outcomes.
Dive into Technavio's robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Celiac Diseases Drugs Market insights. See full methodology.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
212 |
|
Base year |
2024 |
|
Historic period |
2019-2023 |
|
Forecast period |
2025-2029 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 23.6% |
|
Market growth 2025-2029 |
USD 1505 million |
|
Market structure |
Fragmented |
|
YoY growth 2024-2025(%) |
18.9 |
|
Key countries |
US, Canada, Germany, UK, Italy, France, The Netherlands, China, Japan, and India |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
What are the Key Data Covered in this Celiac Diseases Drugs Market Research and Growth Report?
- CAGR of the Celiac Diseases Drugs industry during the forecast period
- Detailed information on factors that will drive the growth and forecasting between 2025 and 2029
- Precise estimation of the size of the market and its contribution of the industry in focus to the parent market
- Accurate predictions about upcoming growth and trends and changes in consumer behaviour
- Growth of the market across North America, Europe, Asia, and Rest of World (ROW)
- Thorough analysis of the market's competitive landscape and detailed information about companies
- Comprehensive analysis of factors that will challenge the celiac diseases drugs market growth of industry companies
We can help! Our analysts can customize this celiac diseases drugs market research report to meet your requirements.