Comprehensive Metabolic Panel Testing Market Size 2025-2029
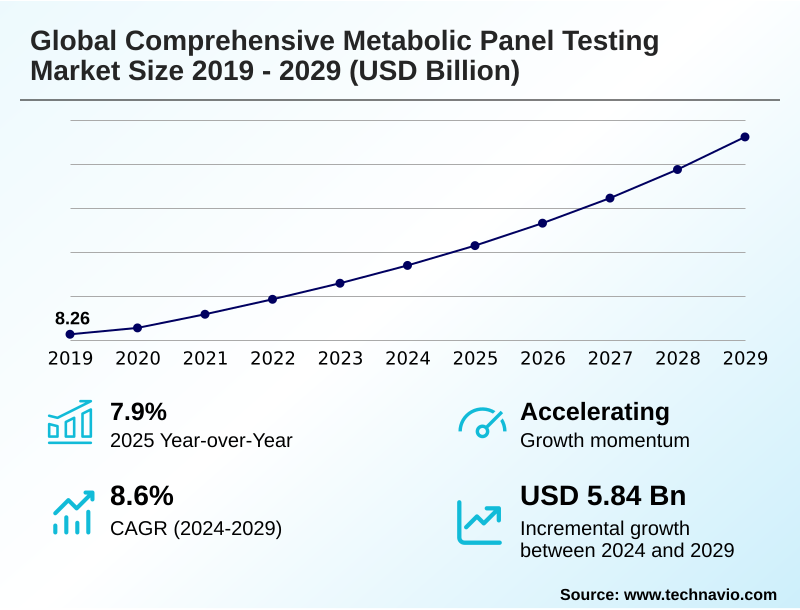
The comprehensive metabolic panel testing market size is valued to increase by USD 5.84 billion, at a CAGR of 8.6% from 2024 to 2029. Escalating incidence of chronic diseases will drive the comprehensive metabolic panel testing market.
Major Market Trends & Insights
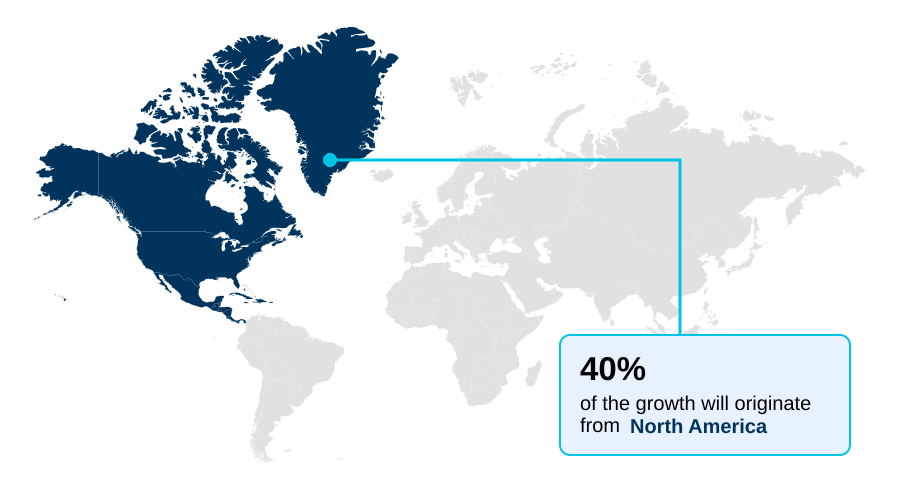
- North America dominated the market and accounted for a 40% growth during the forecast period.
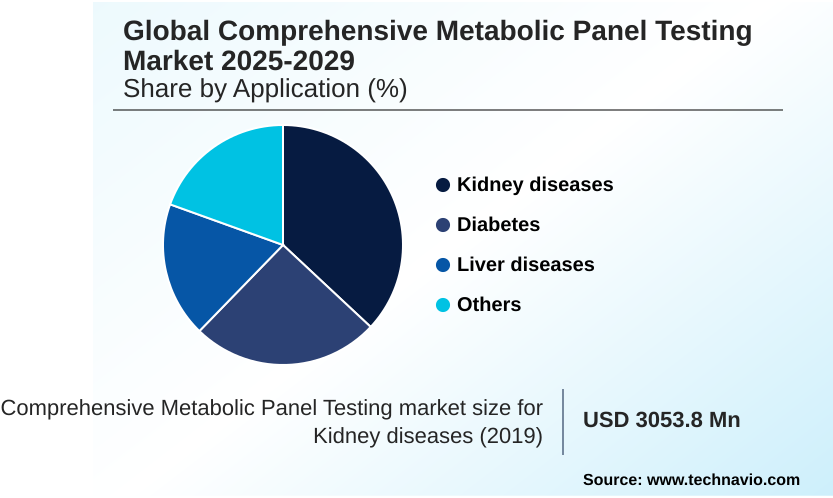
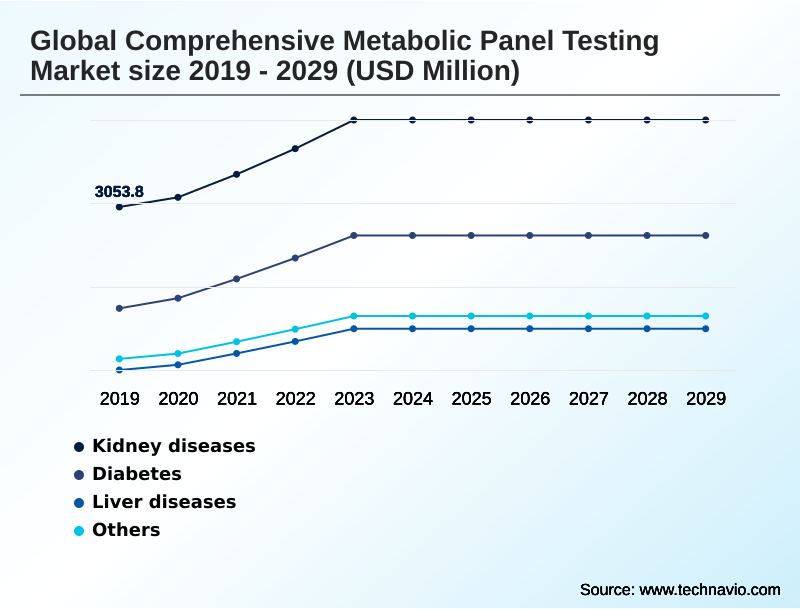
- By Application - Kidney diseases segment was valued at USD 3.88 billion in 2023
- By Test - Kidney function tests segment accounted for the largest market revenue share in 2023
Market Size & Forecast
- Market Opportunities: USD 8.96 billion
- Market Future Opportunities: USD 5.84 billion
- CAGR from 2024 to 2029 : 8.6%
Market Summary
- The comprehensive metabolic panel testing market is fundamental to modern healthcare, providing a broad overview of an individual's metabolic health, including kidney and liver function, electrolyte balance, and blood glucose levels. Growth is driven by the rising prevalence of chronic conditions and an aging population, which demand regular monitoring for effective chronic disease management.
- A significant trend is the shift towards decentralized testing, including point-of-care testing (POCT) and at-home solutions, which offer convenience and rapid results. For instance, a large laboratory network can optimize its diagnostic workflow by deploying CLIA-waived tests in satellite clinics, reducing sample transportation time and enabling faster clinical decision support.
- This enhances operational efficiency by balancing the workload between high-throughput screening in central labs and rapid diagnostics at the periphery. However, the market faces challenges from stringent regulatory compliance frameworks for in-vitro diagnostics (IVD), including laboratory-developed tests (LDTs), and complex reimbursement policies that can limit adoption.
- The integration of artificial intelligence for post-analytical data interpretation and early disease detection presents a major opportunity, promising to improve the predictive power of metabolic assessments.
What will be the Size of the Comprehensive Metabolic Panel Testing Market during the forecast period?
Get Key Insights on Market Forecast (PDF) Request Free Sample
How is the Comprehensive Metabolic Panel Testing Market Segmented?
The comprehensive metabolic panel testing industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2025-2029, as well as historical data from 2019-2023 for the following segments.
- Application
- Kidney diseases
- Diabetes
- Liver diseases
- Others
- Test
- Kidney function tests
- Glucose
- Liver function tests
- Electrolytes
- Others
- End-user
- Laboratories
- Hospital
- Point-of-care testing
- Emergency departments
- Others
- Geography
- North America
- US
- Canada
- Mexico
- Asia
- Europe
- Germany
- UK
- France
- Rest of World (ROW)
- North America
By Application Insights
The kidney diseases segment is estimated to witness significant growth during the forecast period.
The comprehensive metabolic panel testing market application in kidney disease management is expanding, driven by the need for regular monitoring of analytes such as blood urea nitrogen (BUN) and creatinine.
The comprehensive metabolic panel, a key tool in chronic disease management, provides essential data on kidney function through tests for creatinine clearance and estimated glomerular filtration rate (eGFR).
As healthcare systems emphasize early disease detection, the use of a basic metabolic panel alongside the urine albumin-to-creatinine ratio (UACR) is becoming standard. Advances in non-invasive diagnostics and high-throughput screening technologies are improving diagnostic workflow optimization.
This focus on proactive monitoring and value-based healthcare models has led to a 15% improvement in the early detection rates of diabetic nephropathy in targeted population health management programs.
The Kidney diseases segment was valued at USD 3.88 billion in 2023 and showed a gradual increase during the forecast period.
Regional Analysis
North America is estimated to contribute 40% to the growth of the global market during the forecast period.Technavio’s analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
See How Comprehensive Metabolic Panel Testing Market Demand is Rising in North America Request Free Sample
The geographic landscape of the comprehensive metabolic panel testing market is shaped by regional healthcare priorities and technological adoption rates.
In North America, which accounts for approximately 40% of the market's incremental growth, the high prevalence of chronic diseases drives demand. The region's advanced infrastructure supports widespread use of automated chemistry analyzer systems and mass spectrometry platforms for high-throughput screening.
In contrast, markets in Asia are focusing on improving healthcare access through point-of-care testing (POCT) and mobile health initiatives. Europe's market is characterized by stringent regulatory compliance frameworks, such as the IVDR, which influence product development and market entry.
Across all regions, the integration of biosensor technology and microfluidics technology is enabling the development of more compact and efficient diagnostic tools, with some advanced analyzers now capable of processing over 50 key assays in under 14 minutes, enhancing diagnostic test validation and turnaround times.
Market Dynamics
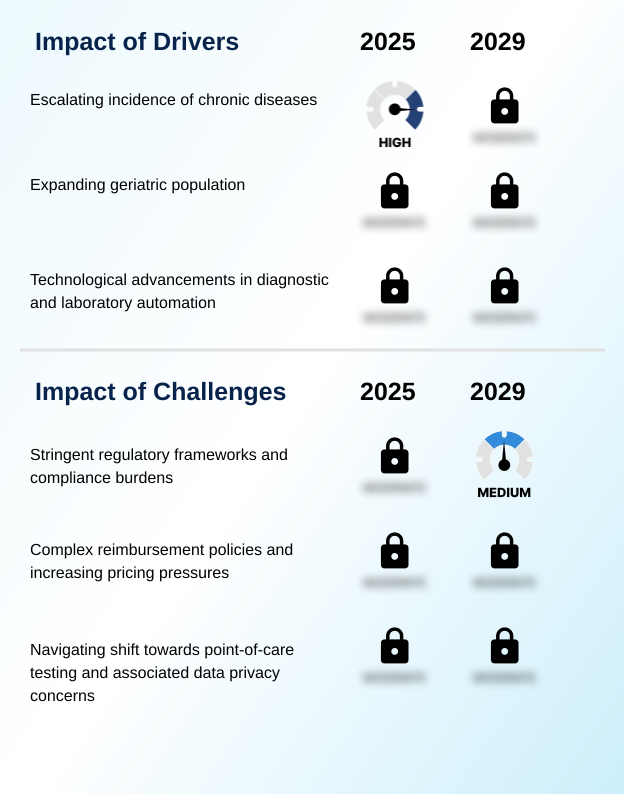
Our researchers analyzed the data with 2024 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
- Strategic focus within the comprehensive metabolic panel testing market is increasingly granular, addressing specific clinical needs and patient pathways. The discussion around a comprehensive vs basic metabolic panel is pivotal in primary care for optimizing diagnostic spending.
- For chronic conditions, the use of a comprehensive metabolic panel for diabetes management and as a metabolic panel for kidney disease is standard practice, with interpretation guides becoming essential clinical tools. The role of liver function tests in cmp is critical for monitoring medication toxicity, while understanding electrolyte imbalance and metabolic panel results is key in acute settings.
- The growth of point-of-care metabolic panel devices and at-home metabolic testing kits is reshaping patient access, raising questions about insurance coverage for cmp testing. The application of ai in metabolic panel analysis promises to revolutionize early detection. In specialized areas, a pediatric comprehensive metabolic panel requires different reference ranges, and metabolic testing for weight loss is a growing consumer trend.
- Clinicians must also consider how to prepare for a cmp test and what to do with abnormal cmp results next, including understanding the impact of medication on cmp.
- In terms of business outcomes, a decentralized testing model using at-home kits has been shown to reduce hospital readmission rates for certain chronic conditions by more than 10% compared to traditional protocols, optimizing resource allocation.
What are the key market drivers leading to the rise in the adoption of Comprehensive Metabolic Panel Testing Industry?
- The escalating incidence of chronic diseases, which necessitate regular monitoring for effective patient management, is a key driver for the comprehensive metabolic panel testing market.
- The market's primary driver is the escalating burden of chronic, lifestyle-associated diseases.
- The high prevalence of these conditions, with nearly half of adults in some developed nations having a cardiovascular disease and nine out of ten older adults reporting at least one chronic illness, creates sustained demand for routine monitoring.
- This demographic reality necessitates a significantly higher frequency of screenings, including kidney function tests and liver function tests, for at-risk populations. This demand underpins the need for efficient population health management strategies and personalized medicine insights.
- The clinical utility of both the comprehensive metabolic panel and specialized in-vitro diagnostics (IVD) in managing conditions like diabetic nephropathy monitoring and cardiovascular risk stratification ensures their central role in the healthcare continuum, driving consistent testing volumes and technological innovation in clinical chemistry assays.
What are the market trends shaping the Comprehensive Metabolic Panel Testing Industry?
- The ascendancy of point-of-care metabolic panel testing signifies a key market trend. This shift is driven by the growing demand for rapid, near-patient analysis in diverse healthcare environments.
- Key market trends are redefining the delivery and utility of metabolic assessments. The proliferation of direct-to-consumer (DTC) testing and at-home solutions, powered by advancements in biosensor technology, is enabling greater patient autonomy in metabolic health assessment.
- The integration of AI into diagnostics, exemplified by tools using over 20 parameters for risk stratification, is enhancing predictive accuracy by more than 15% over traditional methods.
- Concurrently, the adoption of point-of-care testing (POCT) in clinical settings is accelerating, with handheld analyzers delivering lab-quality results for a basic metabolic panel within minutes—a stark contrast to the multi-hour turnaround from centralized labs.
- This shift toward decentralized, technology-driven diagnostics is a cornerstone of modern geriatric medicine screening and remote patient monitoring, supported by robust electronic health record (EHR) integration for seamless data flow.
What challenges does the Comprehensive Metabolic Panel Testing Industry face during its growth?
- Stringent regulatory frameworks and their associated compliance burdens present a key challenge affecting the growth and innovation of the comprehensive metabolic panel testing industry.
- Navigating complex regulatory and data privacy landscapes presents a significant market challenge. Adherence to stringent regulatory compliance frameworks, such as evolving rules for laboratory-developed tests (LDTs) and in-vitro diagnostics (IVD), increases compliance costs for manufacturers by an estimated 20-30%, impacting R&D for new immunoassay systems.
- Furthermore, the rise of direct-to-consumer testing introduces data privacy and security risks, with audits revealing that over 60% of some firms have vague policies, creating challenges for quality management system (QMS) adherence.
- This environment complicates reimbursement policy navigation and requires robust frameworks for diagnostic test validation to ensure both accuracy and security, especially as laboratory information system (LIS) integration becomes more widespread. These hurdles impact the seamless adoption of new technologies and influence overall market accessibility.
Exclusive Technavio Analysis on Customer Landscape
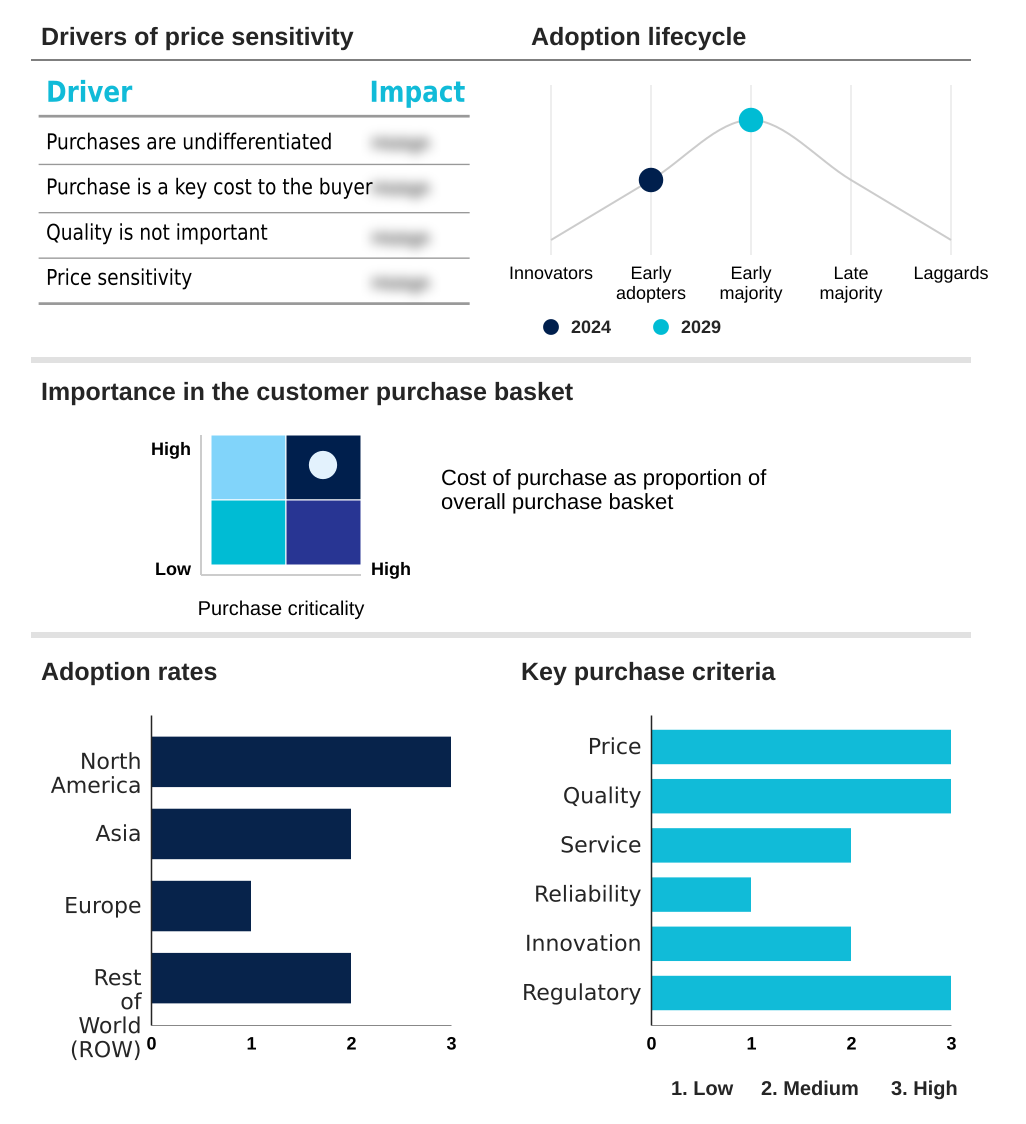
The comprehensive metabolic panel testing market forecasting report includes the adoption lifecycle of the market, covering from the innovator’s stage to the laggard’s stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the comprehensive metabolic panel testing market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape of Comprehensive Metabolic Panel Testing Industry
Competitive Landscape
Companies are implementing various strategies, such as strategic alliances, comprehensive metabolic panel testing market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
Abbott Laboratories - Core laboratory solutions provide essential assays assessing liver, kidney, heart, and metabolic function, forming a foundational component of modern diagnostic services and clinical decision support.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Abbott Laboratories
- ARUP Laboratories
- Beckman Coulter Inc.
- Bio Rad Laboratories Inc.
- CENTOGENE NV
- DiaSorin Spa
- F. Hoffmann La Roche Ltd.
- Laboratory Corp.
- Mayo Medical Laboratories
- Metropolis Healthcare Ltd.
- Quest Diagnostics Inc.
- QuidelOrtho Corp.
- Randox Laboratories Ltd.
- Siemens Healthineers AG
- Sonic Healthcare Ltd.
- SYNLAB International GmbH
- Thermo Fisher Scientific Inc.
- Unipath Specialty Laboratory Ltd.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Comprehensive metabolic panel testing market
- In August, 2024, the U.S. Food and Drug Administration cleared the first over-the-counter continuous glucose biosensor for adults with type 2 diabetes not using insulin, expanding consumer access to metabolic health data.
- In October, 2024, Labcorp announced its role as the exclusive U.S. distributor for the first FDA-authorized over-the-counter syphilis test, a move aimed at broadening the accessibility of critical diagnostics.
- In December, 2024, a partnership was announced between Philips and icometrix to integrate AI-powered brain imaging capabilities, showcasing the trend of incorporating artificial intelligence to enhance diagnostic precision.
- In January, 2025, South Korea's Ministry of Food and Drug Safety implemented the Digital Medical Products Act, establishing a formal approval pathway for AI-enhanced diagnostics and fostering innovation within the sector.
Dive into Technavio’s robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Comprehensive Metabolic Panel Testing Market insights. See full methodology.
| Market Scope | |
|---|---|
| Page number | 308 |
| Base year | 2024 |
| Historic period | 2019-2023 |
| Forecast period | 2025-2029 |
| Growth momentum & CAGR | Accelerate at a CAGR of 8.6% |
| Market growth 2025-2029 | USD 5839.0 million |
| Market structure | Fragmented |
| YoY growth 2024-2025(%) | 7.9% |
| Key countries | US, Canada, Mexico, China, India, Japan, South Korea, Indonesia, Thailand, Singapore, Germany, UK, France, Italy, The Netherlands, Spain, Russia, Australia, Brazil, UAE, South Africa, Saudi Arabia and Turkey |
| Competitive landscape | Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
Research Analyst Overview
- The comprehensive metabolic panel testing market is defined by its critical role in foundational diagnostics, utilizing a range of technologies from clinical chemistry assays to advanced immunoassay systems and molecular diagnostics. The standard comprehensive metabolic panel assesses key indicators like blood glucose levels, creatinine clearance, blood urea nitrogen (BUN), and calcium concentration.
- It includes specific liver function tests such as alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), and total bilirubin, alongside serum albumin levels and total protein measurement to evaluate nutritional status. Electrolyte balance is monitored through sodium levels, potassium concentration, chloride levels, and CO2 (bicarbonate) levels.
- A key boardroom consideration is the capital investment in high-throughput screening systems versus decentralized point-of-care testing (POCT) platforms. The adoption of an advanced automated chemistry analyzer can reduce sample processing time by over 30% compared to manual methods.
- The market is also navigating the regulatory landscape for laboratory-developed tests (LDTs) and CLIA-waived tests, with continuous glucose monitoring (CGM) and non-invasive diagnostics emerging as transformative forces influencing future product strategies.
What are the Key Data Covered in this Comprehensive Metabolic Panel Testing Market Research and Growth Report?
-
What is the expected growth of the Comprehensive Metabolic Panel Testing Market between 2025 and 2029?
-
USD 5.84 billion, at a CAGR of 8.6%
-
-
What segmentation does the market report cover?
-
The report is segmented by Application (Kidney diseases, Diabetes, Liver diseases, and Others), Test (Kidney function tests, Glucose, Liver function tests, Electrolytes, and Others), End-user (Laboratories, Hospital, Point-of-care testing, Emergency departments, and Others) and Geography (North America, Asia, Europe, Rest of World (ROW))
-
-
Which regions are analyzed in the report?
-
North America, Asia, Europe and Rest of World (ROW)
-
-
What are the key growth drivers and market challenges?
-
Escalating incidence of chronic diseases, Stringent regulatory frameworks and compliance burdens
-
-
Who are the major players in the Comprehensive Metabolic Panel Testing Market?
-
Abbott Laboratories, ARUP Laboratories, Beckman Coulter Inc., Bio Rad Laboratories Inc., CENTOGENE NV, DiaSorin Spa, F. Hoffmann La Roche Ltd., Laboratory Corp., Mayo Medical Laboratories, Metropolis Healthcare Ltd., Quest Diagnostics Inc., QuidelOrtho Corp., Randox Laboratories Ltd., Siemens Healthineers AG, Sonic Healthcare Ltd., SYNLAB International GmbH, Thermo Fisher Scientific Inc. and Unipath Specialty Laboratory Ltd.
-
Market Research Insights
- The dynamics of the comprehensive metabolic panel testing market are increasingly shaped by the pursuit of operational efficiency and improved patient outcomes. The adoption of telehealth diagnostic services, for example, has demonstrated the potential to reduce unnecessary screening metabolic panels by over 15% through better patient triage and adherence to medical necessity criteria.
- Concurrently, the integration of point-of-care testing within hospital emergency departments has been linked to a reduction in patient length-of-stay by up to 20%, directly impacting value-based healthcare model metrics. These shifts highlight a strategic pivot towards more targeted and efficient diagnostic workflow optimization.
- As healthcare providers navigate complex reimbursement policy navigation, data-driven approaches that prove clinical and economic value are becoming essential for sustaining growth and justifying investment in advanced diagnostic platforms and remote patient monitoring technologies.
We can help! Our analysts can customize this comprehensive metabolic panel testing market research report to meet your requirements.