Diffuse Large B-Cell Lymphoma (DLBCL) Therapeutics Market Size 2025-2029
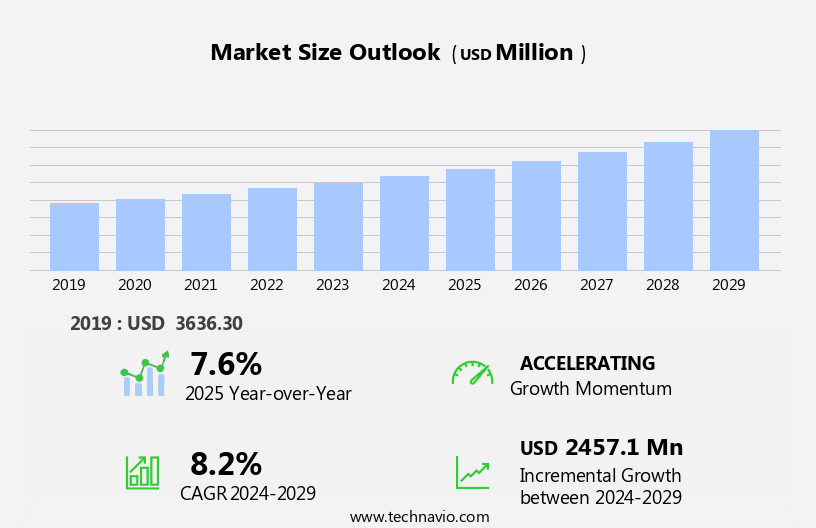
The diffuse large B-Cell lymphoma (DLBCL) therapeutics market size is forecast to increase by USD 2.46 billion, at a CAGR of 8.2% between 2024 and 2029.
- The market is experiencing significant growth, driven primarily by the increasing geriatric population and advancements in technology. The aging demographic is a key factor, as DLBCL is more prevalent in older adults, making up up to 50% of all non-Hodgkin lymphoma diagnoses in this age group. Cell therapy manufacturing and precision medicine are emerging trends, with CAR T-cell therapy and stem cell transplantation offering promising results for refractory DLBCL patients. Moreover, technological innovations are transforming the treatment landscape, with a trend toward less invasive, targeted therapies and the development of new drugs with improved cytotoxicity.
- Companies seeking to capitalize on market opportunities must navigate these challenges by investing in research and development of less toxic therapies and exploring alternative treatment modalities. Additionally, collaboration with regulatory bodies and patient advocacy groups can help ensure the availability and accessibility of effective treatments for this patient population. However, challenges remain, including the cytotoxicity of current drugs and the inclination toward radiation treatments, which can have significant side effects and limit patient compliance. Clinical trial recruitment is streamlined through digital health technologies and patient registries, enabling cost-effective analysis and real-world data collection.
What will be the Size of the Diffuse Large B-Cell Lymphoma (DLBCL) Therapeutics Market during the forecast period?
- The market is experiencing significant advancements, driven by the integration of value-based healthcare and innovative technologies. Antibody-drug conjugates (ADCs) and immunomodulatory drugs are at the forefront of this evolution, targeting specific cell signaling pathways and immune system modulation. Epigenetic therapies and small molecule inhibitors are making strides in addressing tumor heterogeneity, while health economics and data management are crucial in assessing drug safety and cost-effectiveness. Machine learning and artificial intelligence are employed to analyze next-generation sequencing data, enabling outcome measurement and post-marketing surveillance.
- Patient enrollment is optimized through data-driven strategies, and car T-cell engineering offers promising results in personalized treatments. Liquid biopsy and immune system modulation are key areas of focus in the tumor microenvironment, as genetic mutations are identified and targeted. Overall, the DLBCL therapeutics market is dynamic, with continuous innovation in technology and treatment approaches.
How is this Diffuse Large B-Cell Lymphoma (DLBCL) Therapeutics Industry segmented?
The diffuse large B-cell lymphoma (DLBCL) therapeutics industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2025-2029, as well as historical data from 2019-2023 for the following segments.
- Product
- Small molecules
- Biologics
- Application
- Hospital pharmacy
- Retail pharmacy
- Online pharmacy
- Others
- Therapy
- Chemotherapy
- Immunotherapy
- Combination therapies
- Others
- Geography
- North America
- US
- Canada
- Mexico
- Europe
- France
- Germany
- Italy
- UK
- APAC
- China
- India
- Japan
- Rest of World (ROW)
- North America
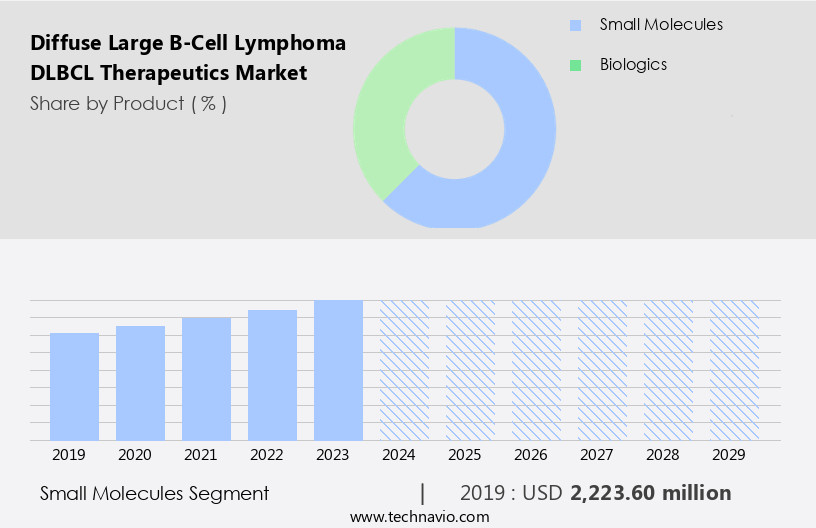
By Product Insights
The small molecules segment is estimated to witness significant growth during the forecast period. The market is witnessing significant growth, driven by advancements in drug development and regulatory approvals. Small molecule drugs, which are low molecular weight organic compounds, play a crucial role in regulating biological processes and are widely used for DLBCL treatment as mono or combination therapy. The market's expansion is fueled by ongoing research in biomarker discovery and genetic profiling, enabling more precise and effective treatments. Regulatory agencies, such as the European Medicines Agency (EMA), are accelerating the approval process for innovative therapies, including immune checkpoint inhibitors and targeted therapies like PD-1 inhibitors and CTL-4 inhibitors. Drug resistance remains a challenge, prompting the development of combination therapies and personalized treatments based on patient genetic profiles.
The market is also witnessing a shift towards targeted therapies and combination treatments, with anti-CD20 antibodies and radiation therapy continuing to be part of the standard of care for DLBCL treatment. The DLBCL therapeutics market is undergoing transformative changes, driven by research, regulatory approvals, and advancements in drug development and manufacturing. The focus on personalized therapy, patient support, and access to treatment is shaping the future of DLBCL care, with the ultimate goal of improving patient outcomes and quality of life.
The Small molecules segment was valued at USD 2.22 billion in 2019 and showed a gradual increase during the forecast period.
Healthcare policy and clinical practice guidelines are evolving to prioritize patient access to advanced treatments and support services, such as patient education, adverse event management, and patient advocacy. Clinical trials are essential for evaluating the safety and efficacy of new therapies, with Phase III trials being a critical stage in the drug development process. Precision medicine aims to tailor treatments based on individual patient characteristics, improving response rates and overall survival. Despite these advancements, challenges persist, including high drug pricing and healthcare costs, disease progression, and the need for improved access to treatment for underserved populations.
Regional Analysis
North America is estimated to contribute 48% to the growth of the global market during the forecast period. Technavio's analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
The market in the US is experiencing significant growth due to the increasing diagnosis of DLBCL, a subtype of Non-Hodgkin Lymphoma (NHL), and the approval of innovative treatments. With over 28.5 million invasive cancer cases diagnosed in the US over the past two decades and over 1 million new cases reported annually, according to the Centers for Disease Control and Prevention (CDC) and the National Program of Cancer Registries (NPCR), the demand for effective DLBCL therapies is on the rise. Biomarker discovery and genetic profiling are crucial in identifying the most effective treatment options for patients.
Regulatory approval of new therapies, such as CTLA-4 inhibitors and immune checkpoint inhibitors, is accelerating, providing doctors with more tools to combat drug resistance. Precision medicine and personalized therapy are becoming the standard of care, with clinical practice guidelines emphasizing the importance of tailoring treatment to individual patients. Clinical trials are ongoing to evaluate the efficacy of combination therapies, such as targeted therapy and radiation therapy, in combination with standard treatments like anti-CD20 antibodies and stem cell transplantation. Phase III trials are investigating the use of CAR T-cell therapy and Brentuximab Vedotin for relapsed and refractory DLBCL.
Healthcare policy and access to treatment are critical factors influencing market dynamics. Patient education and support are essential for ensuring optimal response rates and quality of life. Adverse events and drug pricing are ongoing concerns, with ongoing discussions regarding healthcare costs and treatment affordability. Cell therapy manufacturing and data analysis are essential components of the market, with companies investing in advanced technologies to improve the production and efficacy of therapies. Patient advocacy groups are also playing a vital role in raising awareness and supporting patients through their treatment journey. The DLBCL therapeutics market in the US is evolving rapidly, with a focus on innovation, personalized medicine, and improving patient outcomes.
The market is expected to continue growing, driven by the increasing prevalence of NHL and the ongoing development of new and effective treatments.
Market Dynamics
Our researchers analyzed the data with 2024 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
What are the Diffuse Large B-Cell Lymphoma (DLBCL) Therapeutics market drivers leading to the rise in the adoption of Industry?
- The geriatric population's continued growth serves as the primary market catalyst. The market is experiencing significant growth due to the increasing incidence of this aggressive form of non-Hodgkin lymphoma, particularly among the geriatric population. With aging being a major risk factor for DLBCL, the expanding global population aged 65 and above, notably in developed regions, is driving market demand. This demographic trend necessitates the development of advanced and targeted therapies, such as CAR T-cell therapies and biologics, to cater to the unique needs of elderly patients who often present with comorbidities and reduced tolerance to standard chemotherapy. Data analysis plays a crucial role in identifying effective treatment strategies and improving patient outcomes, including response rate, overall survival, and quality of life.
- Patient advocacy groups are emphasizing the importance of these factors, further fueling the market's growth. Anti-CD20 antibodies, which have shown promising results in DLBCL treatment, have received EMA approval, contributing to the market's expansion. The focus on innovative therapies and improving patient care underscores the potential for substantial growth in the DLBCL therapeutics market.
What are the Diffuse Large B-Cell Lymphoma (DLBCL) Therapeutics market trends shaping the Industry?
- The current market trend is shaped by significant technological advances. It is essential to stay informed about these innovations to maintain a competitive edge. Technological progress is driving the market forward, making it crucial for professionals to keep abreast of the latest developments. DLBCL, a type of non-Hodgkin's lymphoma, poses significant challenges in cancer treatment. Current therapies include radiation therapy, bone marrow transplant, and chemotherapy. However, relapsed DLBCL often requires new approaches.
- Combination therapies and updated treatment guidelines aim to improve patient outcomes while managing healthcare costs. The recent research advances offer hope for those battling DLBCL, emphasizing the need for continued investment in research and development. Innovative treatments, such as CAR T-cell therapy, gene therapy, companion diagnostics, and personalized medicines, are under development. For instance, Yescarta, a CAR T-cell immune therapy by Gilead, and Kymriah (tisagenlecleucel) from Novartis, are first-in-class treatments for refractory large B-cell lymphoma. Additionally, targeted therapies like brentuximab vedotin and PD-1 inhibitors are showing promise in clinical trials.
How does Diffuse Large B-Cell Lymphoma (DLBCL) Therapeutics market face challenges during its growth?
- The cytotoxicity of drugs and the propensity for radiation therapies pose a significant challenge to the growth of the industry, as effective management of these treatments is crucial for patient safety and therapeutic success. Diffuse Large B-Cell Lymphoma (DLBCL) is a type of non-Hodgkin's lymphoma (NHL) that requires effective therapeutic interventions due to its aggressive nature and potential for drug resistance. Biomarker discovery and genetic profiling are crucial in identifying personalized treatment approaches, leading to improved progression-free survival (PFS) and partial remission rates. Regulatory approval of novel therapies, such as CTLA-4 inhibitors and precision medicine, is essential in addressing treatment resistance and enhancing clinical practice guidelines.
- The healthcare policy landscape plays a significant role in shaping the DLBCL therapeutics market, influencing access to advanced treatments and driving research and development efforts. The focus on precision medicine and immunotherapies, such as car T-cell therapy, is expected to revolutionize the treatment landscape and improve patient outcomes. Drug development is ongoing to mitigate side effects, including cytotoxicity, which can impact patient quality of life. Radiation therapy, though effective for some NHL types, may not be suitable for all DLBCL cases due to its potential side effects and limited applicability.
Exclusive Customer Landscape
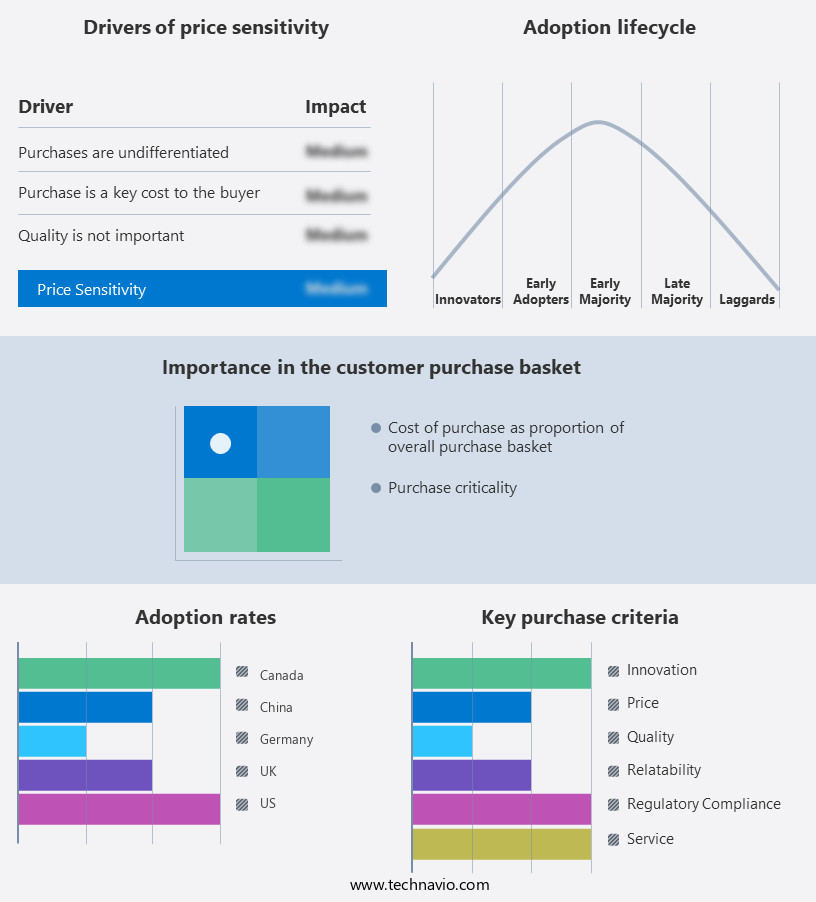
The diffuse large B-cell lymphoma (DLBCL) therapeutics market forecasting report includes the adoption lifecycle of the market, covering from the innovator's stage to the laggard's stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the diffuse large B-cell lymphoma (DLBCL) therapeutics market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape
Key Companies & Market Insights
Companies are implementing various strategies, such as strategic alliances, diffuse large B-cell lymphoma (dlbcl) therapeutics market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
AbbVie Inc. - The company specializes in the development of innovative therapeutics for diffuse large B-cell lymphoma, including DuoBody CD3xCD20.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- AbbVie Inc.
- ADC Therapeutics SA
- Amgen Inc.
- Bayer AG
- BeiGene Ltd.
- Bristol Myers Squibb Co.
- Celltrion Healthcare Co. Ltd.
- Erytech Pharma SA
- F. Hoffmann La Roche Ltd.
- Gilead Sciences Inc.
- GlaxoSmithKline Plc
- Johnson and Johnson Services Inc.
- Merck and Co. Inc.
- Novartis AG
- Pfizer Inc.
- Seagen Inc.
- Spectrum Pharmaceuticals Inc.
- Swedish Orphan Biovitrum AB
- Takeda Pharmaceutical Co. Ltd.
- Teva Pharmaceutical Industries Ltd.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Diffuse Large B-Cell Lymphoma (DLBCL) Therapeutics Market
- In February 2023, Roche's Kadcyla, in combination with Tecfidera from Biogen, received a new indication for the treatment of certain types of DLBCL, marking a significant advancement in targeted therapy for this disease (Roche Press Release, 2023).
- In March 2024, Merck KGaA and Incyte Corporation announced a strategic collaboration to develop and commercialize epacadostat, an IDO1 inhibitor, in combination with Merck KGaA's anti-PD-L1 therapy, avelumab, for the treatment of relapsed or refractory DLBCL. This partnership could potentially lead to more effective immunotherapies for DLBCL patients (Merck KGaA Press Release, 2024).
- In May 2024, the FDA granted accelerated approval to Selinexor from Karyopharm Therapeutics in combination with dexamethasone for the treatment of patients with relapsed or refractory DLBCL who have received at least two prior therapies. This marks the first approval of an oral Selective Inhibitor of Nuclear Export (SINE) compound for DLBCL (FDA Press Release, 2024).
Research Analyst Overview
The market continues to evolve as new treatments and therapies emerge, responding to the dynamic nature of this complex disease. Radiation therapy and bone marrow transplant remain foundational treatments for DLBCL, but their applications are constantly evolving. For instance, advances in radiation therapy technology enable more precise and effective treatments, while advances in bone marrow transplant techniques allow for greater access and improved outcomes for patients. Relapsed DLBCL poses a significant challenge, leading to the development of novel therapies such as Brentuximab Vedotin and PD-1 inhibitors. These targeted therapies, along with combination therapies, offer new hope for patients experiencing disease progression.
Treatment guidelines continue to evolve, reflecting the latest research and clinical findings. Healthcare costs remain a critical concern in the DLBCL therapeutics market. The high cost of new treatments and therapies, such as CAR T-cell therapy and PD-1 inhibitors, can limit access for some patients. Ongoing efforts to improve healthcare policy and patient education aim to address these challenges, ensuring that patients receive the best possible care. The DLBCL therapeutics market is characterized by continuous innovation and the integration of new technologies, such as genetic profiling and precision medicine. Phase III trials and data analysis play a crucial role in bringing new treatments to market, while patient advocacy and personalized therapy initiatives prioritize patient needs and quality of life.
Adverse events and drug pricing remain important considerations in the DLBCL therapeutics market. The development of targeted therapies and combination therapies offers potential solutions to drug resistance and the need for standard of care treatments that provide better response rates and overall survival. The manufacturing and production of cell therapies, such as CAR T-cell therapy and stem cell transplantation, continue to be refined to improve access and affordability. In summary, the DLBCL therapeutics market is characterized by ongoing innovation and the integration of new technologies and therapies. The evolving nature of this market requires a dynamic approach to understanding its applications across various sectors and the continuous unfolding of market activities.
Radiation therapy, bone marrow transplant, relapsed DLBCL, Brentuximab Vedotin, disease progression, combination therapy, treatment guidelines, healthcare costs, PD-1 inhibitors, targeted therapy, and other emerging trends all contribute to the complexity and importance of this market.
Dive into Technavio's strong research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Diffuse Large B-Cell Lymphoma (DLBCL) Therapeutics Market insights. See full methodology.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
220 |
|
Base year |
2024 |
|
Historic period |
2019-2023 |
|
Forecast period |
2025-2029 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 8.2% |
|
Market growth 2025-2029 |
USD 2.46 billion |
|
Market structure |
Fragmented |
|
YoY growth 2024-2025(%) |
7.6 |
|
Key countries |
US, Canada, Germany, China, UK, France, Japan, Mexico, Italy, and India |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
What are the Key Data Covered in this Diffuse Large B-Cell Lymphoma (DLBCL) Therapeutics Market Research and Growth Report?
- CAGR of the Diffuse Large B-Cell Lymphoma (DLBCL) Therapeutics industry during the forecast period
- Detailed information on factors that will drive the growth and forecasting between 2025 and 2029
- Precise estimation of the size of the market and its contribution of the industry in focus to the parent market
- Accurate predictions about upcoming growth and trends and changes in consumer behaviour
- Growth of the market across North America, Europe, Asia, and Rest of World (ROW)
- Thorough analysis of the market's competitive landscape and detailed information about companies
- Comprehensive analysis of factors that will challenge the diffuse large b-cell lymphoma (dlbcl) therapeutics market growth of industry companies
We can help! Our analysts can customize this diffuse large b-cell lymphoma (dlbcl) therapeutics market research report to meet your requirements.