Duchenne Muscular Dystrophy (DMD) Therapeutics Market Size 2026-2030
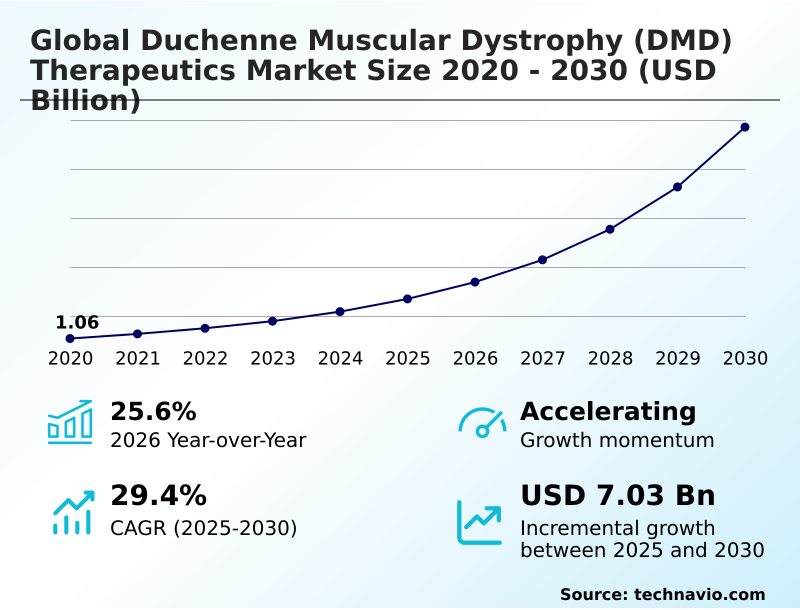
The duchenne muscular dystrophy (dmd) therapeutics market size is valued to increase by USD 7.03 billion, at a CAGR of 29.4% from 2025 to 2030. Commercialization and label expansion of gene therapies will drive the duchenne muscular dystrophy (dmd) therapeutics market.
Major Market Trends & Insights
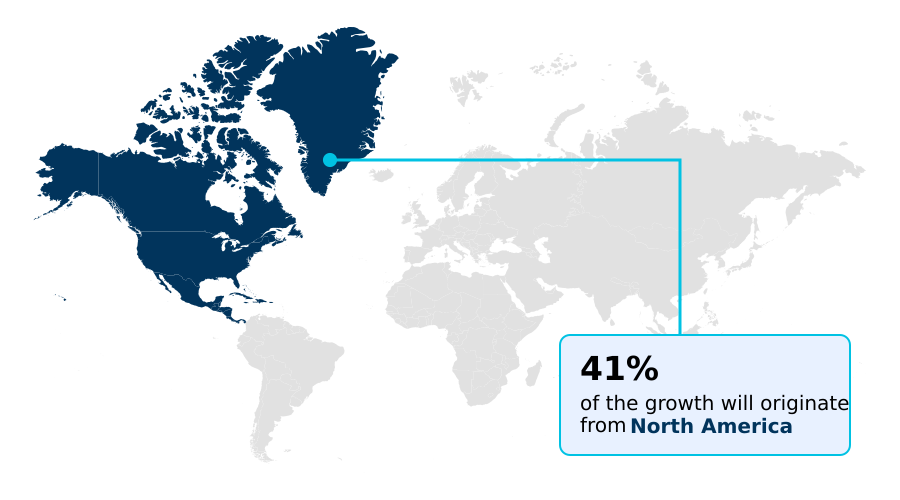
- North America dominated the market and accounted for a 40.8% growth during the forecast period.
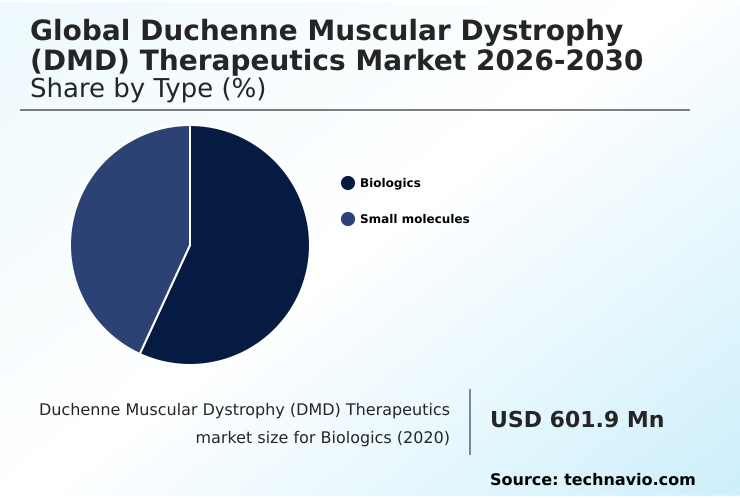
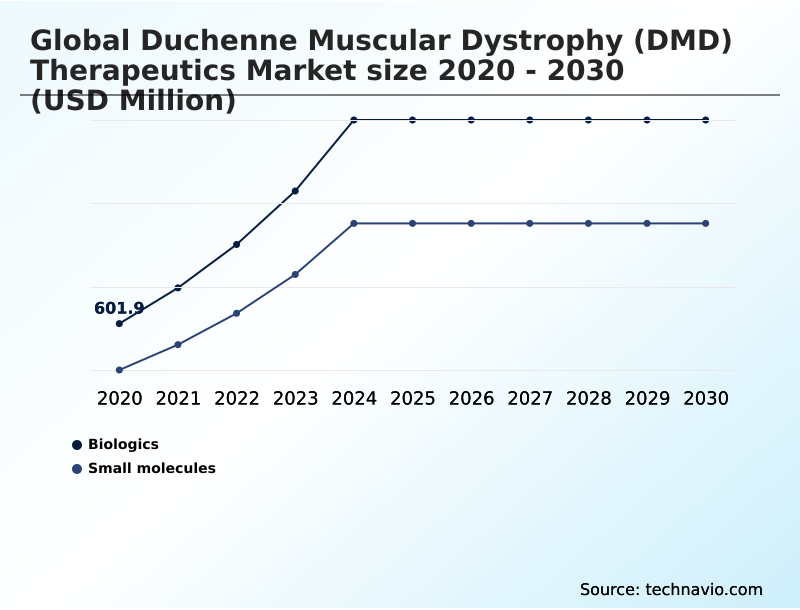
- By Type - Biologics segment was valued at USD 1.24 billion in 2024
- By Distribution Channel - Offline segment accounted for the largest market revenue share in 2024
Market Size & Forecast
- Market Opportunities: USD 8.65 billion
- Market Future Opportunities: USD 7.03 billion
- CAGR from 2025 to 2030 : 29.4%
Market Summary
- The Duchenne Muscular Dystrophy (DMD) therapeutics market is undergoing a significant transformation, driven by a paradigm shift from palliative care to disease-modifying interventions. Central to this evolution is the advancement of AAV vector-based therapies and exon-skipping oligonucleotides, which target the underlying genetic defects of the disease.
- The approval of novel non-steroidal DMD treatment options, such as histone deacetylase inhibitors, diversifies the therapeutic landscape, offering alternatives to corticosteroids and their associated side effects. A key business scenario involves navigating the complex reimbursement environment for high-cost gene therapy for DMD.
- Manufacturers must develop value-based contracts with payers, linking payment to long-term clinical outcomes to ensure patient access while managing financial risk for healthcare systems. This requires robust data on dystrophin restoration therapies and their durable impact on muscle function.
- However, challenges persist, including the high rate of clinical trial failures and regulatory divergences between major markets, which can delay the availability of new DMD therapies. The field is also seeing progress in peptide-conjugated oligonucleotides and other next-generation platforms designed to enhance drug delivery and efficacy, indicating a robust pipeline aimed at addressing this unmet medical need.
What will be the Size of the Duchenne Muscular Dystrophy (DMD) Therapeutics Market during the forecast period?
Get Key Insights on Market Forecast (PDF) Request Free Sample
How is the Duchenne Muscular Dystrophy (DMD) Therapeutics Market Segmented?
The duchenne muscular dystrophy (dmd) therapeutics industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2026-2030, as well as historical data from 2020-2024 for the following segments.
- Type
- Biologics
- Small molecules
- Distribution channel
- Offline
- Online
- Product type
- Corticosteroids
- Pain management drugs
- Geography
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Asia
- Rest of World (ROW)
- North America
By Type Insights
The biologics segment is estimated to witness significant growth during the forecast period.
The biologics segment represents the forefront of disease-modifying interventions in the Duchenne muscular dystrophy therapeutics market, shifting focus from symptomatic management to genetic correction.
This category is dominated by AAV vector-based therapies, exon-skipping oligonucleotides, and antibody-oligonucleotide conjugates designed to restore functional dystrophin. Gene therapy for DMD, particularly micro-dystrophin gene therapy, is a cornerstone, utilizing viral vectors to address the root genetic defect.
The development of these complex large molecules, including cardiosphere-derived cells, involves sophisticated manufacturing and requires administration in specialized centers.
The clinical efficacy of these dystrophin restoration therapies has been validated, with some platforms demonstrating dystrophin expression levels substantially exceeding previous standards, marking a significant advancement in the treatment paradigm.
The Biologics segment was valued at USD 1.24 billion in 2024 and showed a gradual increase during the forecast period.
Regional Analysis
North America is estimated to contribute 40.8% to the growth of the global market during the forecast period.Technavio’s analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
See How Duchenne Muscular Dystrophy (DMD) Therapeutics Market Demand is Rising in North America Request Free Sample
The geographic landscape of the market is led by North America, which accounts for over 40% of the incremental growth, underpinned by the US market's rapid adoption of new DMD therapies.
The region's leadership is driven by a favorable regulatory environment and high investment in R&D for nonsense mutation therapy and other advanced treatments. Europe follows, with a complex reimbursement environment that creates staggered market access.
However, Asia is projected to be the fastest-growing region, with a CAGR of 30.0%.
This growth is fueled by regulatory harmonization and the strategic entry of global players into markets like Japan and China, which are increasingly approving corticosteroid alternatives for DMD.
This regional dynamic is reshaping clinical trial strategies, with a greater emphasis on generating global data to support approvals in emerging markets and advance dystrophin restoration therapies worldwide.
Market Dynamics
Our researchers analyzed the data with 2025 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
- The evolution of the global Duchenne Muscular Dystrophy (DMD) therapeutics market 2026-2030 is marked by significant scientific and commercial complexities. A primary concern is the high cost of gene therapy for Duchenne Muscular Dystrophy, which poses substantial reimbursement challenges for healthcare systems globally and prompts debate on value-based pricing models for dmd drugs.
- Simultaneously, the long-term side effects of corticosteroids in dmd treatment continue to drive research into safer alternatives. The market's trajectory is heavily influenced by the latest clinical trial results for dmd therapies, where both landmark successes and high-profile failures shape investment and R&D strategies.
- For instance, the number of successful late-stage trials for gene therapies is still significantly lower than for small-molecule drugs, impacting operational planning for manufacturing scale-up. Navigating the regulatory approval process for dmd drugs remains a critical hurdle, with notable divergence in evidentiary requirements between US and EU authorities, particularly concerning the acceptance of surrogate endpoints for dmd therapies.
- This regulatory landscape directly impacts the comparison of different dmd treatment options available to patients. Advances in exon skipping technology for dmd and the development of next-generation gene replacement therapies for muscular dystrophy are at the forefront of innovation, promising enhanced efficacy.
- The role of dystrophin levels as a biomarker in dmd trials continues to be a pivotal topic, influencing both drug development and regulatory decisions. Exploring the benefits and risks of emerging dmd treatment pipelines is crucial for stakeholders, as is understanding the effectiveness of non-pharmacological therapies for duchenne.
- Additionally, assessing patient quality of life with new dmd treatments is becoming a key metric for demonstrating therapeutic value beyond functional endpoints.
What are the key market drivers leading to the rise in the adoption of Duchenne Muscular Dystrophy (DMD) Therapeutics Industry?
- The commercialization and subsequent label expansion of novel gene therapies is a primary driver fueling market growth and shifting the treatment paradigm toward disease-modifying interventions.
- Market growth is primarily driven by the successful commercialization and label expansion of transformative gene therapies.
- The expanded US approval of Elevidys to include a broader patient population, now covering non-ambulatory individuals, has substantially increased the addressable market, driving significant revenue growth with its one-time treatment model.
- This success serves as a critical benchmark, stimulating further investment in AAV vector-based therapies. Another major driver is the approval of novel non-steroidal DMD treatment options, such as the histone deacetylase inhibitor Duvyzat.
- As the first drug authorized for all genetic variants, it diversifies the therapeutic arsenal and enables combination treatment strategies, increasing the volume of therapeutics used per patient.
- Strategic global partnerships are also fueling expansion, with collaborations facilitating regulatory submissions in key Asian markets like Japan, which is accelerating global access to advanced therapies.
What are the market trends shaping the Duchenne Muscular Dystrophy (DMD) Therapeutics Industry?
- A key market trend is the expansion of dissociative steroid therapies. These next-generation treatments are gaining prominence for offering improved safety profiles compared to traditional corticosteroids.
- A key trend shaping the market is the rapid advancement beyond first-generation technologies toward more efficient and safer treatment modalities. The expansion of dissociative steroids, such as vamorolone, is gaining momentum, with approvals in major markets like China offering a significant improvement over traditional corticosteroids by minimizing side effects.
- This has led to better patient adherence, with some studies showing a 15% lower discontinuation rate compared to conventional steroids. Concurrently, the evolution toward next-generation delivery systems, including peptide-conjugated oligonucleotides, is enhancing the efficacy of exon-skipping drugs. These platforms achieve higher levels of dystrophin expression with less frequent dosing, improving functional outcomes and justifying premium pricing to payers.
- Furthermore, regulatory acceptance of gene therapy for DMD in emerging regions like Latin America is broadening patient access and setting new precedents for global commercialization strategies.
What challenges does the Duchenne Muscular Dystrophy (DMD) Therapeutics Industry face during its growth?
- The high cost of treatment and the associated reimbursement barriers present a significant challenge affecting industry growth and patient access to innovative therapies.
- The market faces significant challenges, primarily the exorbitant cost of novel treatments and the resulting reimbursement barriers. Gene therapies launched with multi-million-dollar price tags create substantial financial strain on healthcare systems, leading to restrictive payer policies and unequal patient access, where approval does not guarantee immediate treatment.
- Regulatory divergence is another major hurdle; for instance, the European Medicines Agency's demand for functional clinical proof contrasts with the US FDA's acceptance of surrogate endpoints, complicating global trial design and delaying access for European patients. A single negative opinion from a major regulatory body can impact market confidence.
- Furthermore, the high rate of clinical trial failures, such as the setback in a late-stage confirmatory trial for an exon-skipping therapy, underscores the scientific complexity of the disease and the financial risks of development.
Exclusive Technavio Analysis on Customer Landscape
The duchenne muscular dystrophy (dmd) therapeutics market forecasting report includes the adoption lifecycle of the market, covering from the innovator’s stage to the laggard’s stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the duchenne muscular dystrophy (dmd) therapeutics market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape of Duchenne Muscular Dystrophy (DMD) Therapeutics Industry
Competitive Landscape
Companies are implementing various strategies, such as strategic alliances, duchenne muscular dystrophy (dmd) therapeutics market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
Akashi Therapeutics, Inc. - Key offerings include advanced RNA-based therapeutics, pioneering antibody-oligonucleotide conjugates designed to target and treat rare neuromuscular genetic disorders with high precision.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Akashi Therapeutics, Inc.
- Avidity Biosciences, Inc.
- BioMarin Pharmaceutical Inc.
- Capricor Therapeutics Inc.
- Editas Medicine, Inc.
- F. Hoffmann La Roche Ltd.
- FibroGen Inc.
- Italfarmaco Holding SPA
- MeiraGTx Holdings Plc.
- NS Pharma, Inc.
- Precision BioSciences, Inc.
- PTC Therapeutics Inc.
- ReveraGen BioPharma, Inc.
- Sarepta Therapeutics Inc.
- Ultragenyx Pharmaceutical Inc
- Wave Life Sciences Ltd.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Duchenne muscular dystrophy (dmd) therapeutics market
- In August, 2024, Chugai Pharmaceutical Co., Ltd. filed a regulatory application with Japan's Ministry of Health, Labour and Welfare for the approval of delandistrogene moxeparvovec (Elevidys) to treat DMD, expanding its potential reach into the Asian market.
- In September, 2024, Dyne Therapeutics announced positive initial clinical data from its Phase 1/2 DELIVER trial of DYNE-251, a peptide-conjugated oligonucleotide, showing significant dystrophin expression in patients with DMD amenable to exon 51 skipping.
- In December, 2024, China's National Medical Products Administration (NMPA) approved Agamree (vamorolone) for treating DMD, making it the first dissociative steroid available for this indication in the country.
- In December, 2024, the Brazilian Health Regulatory Agency (ANVISA) granted registration approval for Elevidys (delandistrogene moxeparvovec), marking the first gene therapy for DMD authorized in Latin America.
Dive into Technavio’s robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Duchenne Muscular Dystrophy (DMD) Therapeutics Market insights. See full methodology.
| Market Scope | |
|---|---|
| Page number | 275 |
| Base year | 2025 |
| Historic period | 2020-2024 |
| Forecast period | 2026-2030 |
| Growth momentum & CAGR | Accelerate at a CAGR of 29.4% |
| Market growth 2026-2030 | USD 7030.1 million |
| Market structure | Fragmented |
| YoY growth 2025-2026(%) | 25.6% |
| Key countries | US, Canada, Mexico, Germany, UK, France, Italy, Spain, The Netherlands, China, Japan, India, South Korea, Indonesia, Thailand, Brazil, South Africa, Australia, Argentina, Saudi Arabia, Nigeria, UAE and Colombia |
| Competitive landscape | Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
Research Analyst Overview
- The Duchenne Muscular Dystrophy therapeutics market is pivoting from symptomatic management to fundamentally disease-modifying interventions. This shift is powered by breakthroughs in genetic correction, including in-vivo gene-editing and advanced AAV vector-based therapies. The approval of the first micro-dystrophin gene therapy has established a new clinical benchmark, compelling a strategic re-evaluation of R&D portfolios across the industry.
- For boardroom-level planning, this transition necessitates a focus on high-risk, high-reward investments in platforms like exon-skipping oligonucleotides and peptide-conjugated oligonucleotides. The commercial viability of these treatments, underscored by the fact that biologics now command a significantly larger market share than small molecules, is reshaping financial models. The emergence of cardiosphere-derived cells and antibody-oligonucleotide conjugates further diversifies the pipeline.
- However, the development of these muscle-protective agents and RNA-based therapies is constrained by complex manufacturing processes and stringent regulatory hurdles. Success hinges on navigating these challenges while demonstrating clear value through durable clinical outcomes, moving beyond traditional nonsense mutation therapy approaches.
What are the Key Data Covered in this Duchenne Muscular Dystrophy (DMD) Therapeutics Market Research and Growth Report?
-
What is the expected growth of the Duchenne Muscular Dystrophy (DMD) Therapeutics Market between 2026 and 2030?
-
USD 7.03 billion, at a CAGR of 29.4%
-
-
What segmentation does the market report cover?
-
The report is segmented by Type (Biologics, and Small molecules), Distribution Channel (Offline, and Online), Product Type (Corticosteroids, and Pain management drugs) and Geography (North America, Europe, Asia, Rest of World (ROW))
-
-
Which regions are analyzed in the report?
-
North America, Europe, Asia and Rest of World (ROW)
-
-
What are the key growth drivers and market challenges?
-
Commercialization and label expansion of gene therapies, High cost of treatment and reimbursement barriers
-
-
Who are the major players in the Duchenne Muscular Dystrophy (DMD) Therapeutics Market?
-
Akashi Therapeutics, Inc., Avidity Biosciences, Inc., BioMarin Pharmaceutical Inc., Capricor Therapeutics Inc., Editas Medicine, Inc., F. Hoffmann La Roche Ltd., FibroGen Inc., Italfarmaco Holding SPA, MeiraGTx Holdings Plc., NS Pharma, Inc., Precision BioSciences, Inc., PTC Therapeutics Inc., ReveraGen BioPharma, Inc., Sarepta Therapeutics Inc., Ultragenyx Pharmaceutical Inc and Wave Life Sciences Ltd.
-
Market Research Insights
- Market dynamics are shaped by the rapid adoption of innovative treatments and evolving regulatory landscapes. The successful commercialization of gene therapy for DMD has set a new benchmark, compelling competitors to accelerate development of next-generation platforms.
- A significant portion of market momentum, over 40% of the incremental growth, originates from North America due to its favorable regulatory pathways and high healthcare spending. However, the market in Asia is expanding at the fastest rate, with a regional CAGR of 30.0%, driven by improving healthcare access and strategic partnerships.
- This geographical shift is creating new opportunities and challenges related to pricing and market access for new DMD therapies. Payers are increasingly scrutinizing the cost-effectiveness of dystrophin restoration therapies, leading to the implementation of value-based reimbursement models to manage the high cost of treatment and ensure sustainable patient access.
We can help! Our analysts can customize this duchenne muscular dystrophy (dmd) therapeutics market research report to meet your requirements.