Liposomal Products Market Size 2024-2028
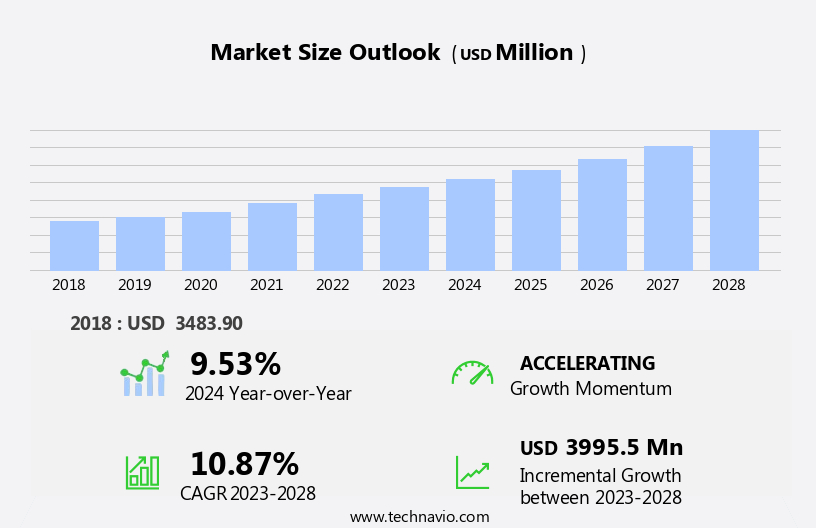
The liposomal products market size is forecast to increase by USD 4 billion, at a CAGR of 10.87% between 2023 and 2028.
- The market is experiencing significant growth, driven by the rising incidence of fungal disorders and the increasing adoption of quality by design (QbD) over traditional manufacturing processes. The prevalence of fungal disorders is escalating due to factors such as an aging population, rising urbanization, and improved diagnosis techniques. This trend presents a substantial opportunity for market participants to develop innovative liposomal products to address unmet medical needs. However, the market faces challenges, including inadequate reimbursement policies for cancer treatment and liposomal products.
- The lack of adequate reimbursement poses a significant barrier to market entry for new players and restricts the growth potential of existing ones. To navigate this challenge, companies must focus on demonstrating the clinical and cost-effectiveness of their liposomal products to healthcare providers and payers. By doing so, they can differentiate their offerings and secure favorable reimbursement policies, thereby expanding their market reach and revenue potential.
What will be the Size of the Liposomal Products Market during the forecast period?
Explore in-depth regional segment analysis with market size data - historical 2018-2022 and forecasts 2024-2028 - in the full report.
Request Free Sample
The market continues to evolve, driven by advancements in transdermal delivery and injectable formulations. Liposomes, nanoscale vesicles made of phospholipids, offer enhanced drug delivery capabilities through various sectors. Size distribution and surface modification play crucial roles in optimizing liposome performance, ensuring stability during storage and improving encapsulation efficiency. Liposomal encapsulation has expanded beyond pharmaceuticals to dietary supplements, leveraging the benefits of bioavailability enhancement. Drug loading capacity is a significant factor in the development of liposomal formulations, with clinical trials exploring the potential of lipid nanoparticles in targeted drug delivery. Stealth liposomes, characterized by their ability to evade the reticuloendothelial system, have gained attention for their potential in intravenous administration and controlled release applications.
Ongoing research focuses on the manufacturing process, biocompatible materials, and steric stabilization for sustained release and intramuscular injection formulations. Anionic and cationic liposomes have distinct applications in drug delivery systems, with the former used for gene therapy and the latter for vaccine development. The market dynamics continue to unfold, with polymeric liposomes and topical applications emerging as promising areas for innovation. Release kinetics and shelf life remain critical considerations in the development and commercialization of liposomal products. As the market evolves, it is essential to stay informed of the latest trends, applications, and technological advancements.
How is this Liposomal Products Industry segmented?
The liposomal products industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2024-2028, as well as historical data from 2018-2022 for the following segments.
- Application
- Cancer
- Fungal infections
- Pain management
- Viral vaccines
- Photodynamic therapy
- Type
- Branded
- Generic
- Geography
- North America
- US
- Europe
- France
- Germany
- APAC
- China
- Japan
- Rest of World (ROW)
- North America
By Application Insights
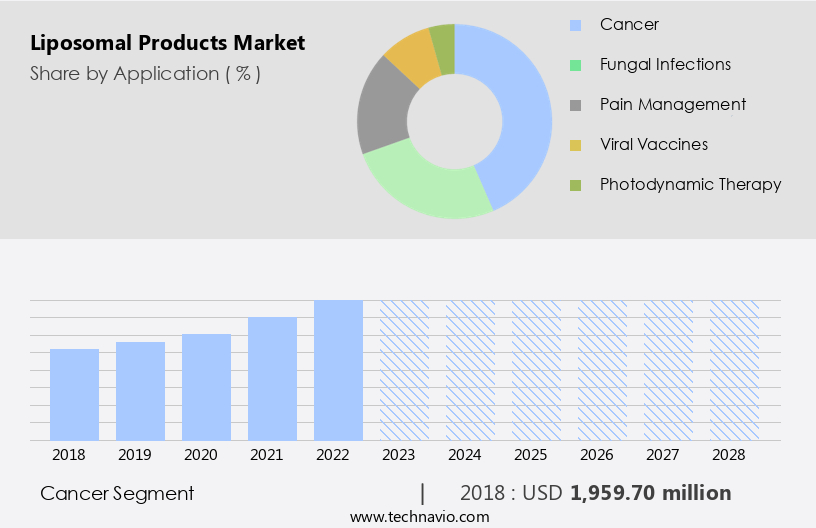
The cancer segment is estimated to witness significant growth during the forecast period.
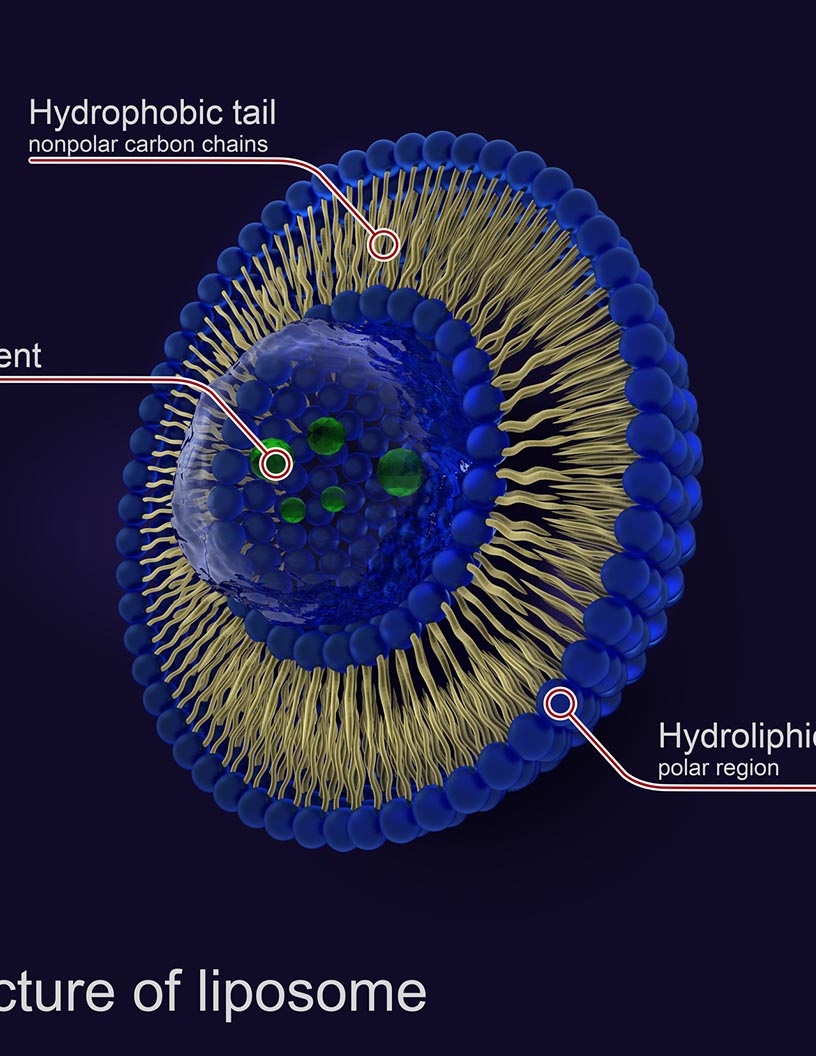
Liposomal products have gained significant attention in the healthcare industry, particularly in cancer therapy, due to their ability to provide targeted drug delivery and controlled release. This property is crucial for effective cancer treatment, as it increases the efficacy of drugs while reducing toxic side effects. Doxil, a PEGylated liposomal formulation encapsulating doxorubicin, is a prime example. Approved by the FDA for treating Kaposis sarcoma in AIDS patients, Doxil is a commercial success, marketed by Johnson and Johnson under the names Doxil in the US and Caelyx outside of it. The advancements in liposomal vesicle development have led to the creation of biocompatible materials for liposomal encapsulation, ensuring improved encapsulation efficiency and drug loading capacity.
Size distribution and surface modification play a vital role in the manufacturing process, ensuring storage stability and steric stabilization. These lipid nanoparticles are also used in veterinary medicine and topical applications. Intravenous, intramuscular, and subcutaneous injections are common methods of administering liposomal products. Anionic and cationic liposomes are used for various drug delivery systems, while polymeric liposomes are used for sustained release. Clinical trials are ongoing to explore the potential of liposomal products in intravenous administration and transdermal delivery. Liposomal products' shelf life is a critical factor, with quality control measures ensuring their stability during storage. Stealth liposomes, which have a long circulation time, are used to improve bioavailability enhancement.
Release kinetics is another essential aspect, with controlled and sustained release properties ensuring the drug's efficacy over an extended period. In summary, the market is evolving, with a focus on improving drug delivery systems, reducing side effects, and enhancing therapeutic efficacy. The use of liposomes in cancer therapy, veterinary medicine, and topical applications is gaining momentum, with ongoing research exploring new possibilities in intravenous administration and transdermal delivery.
The Cancer segment was valued at USD 1.96 billion in 2018 and showed a gradual increase during the forecast period.
Regional Analysis
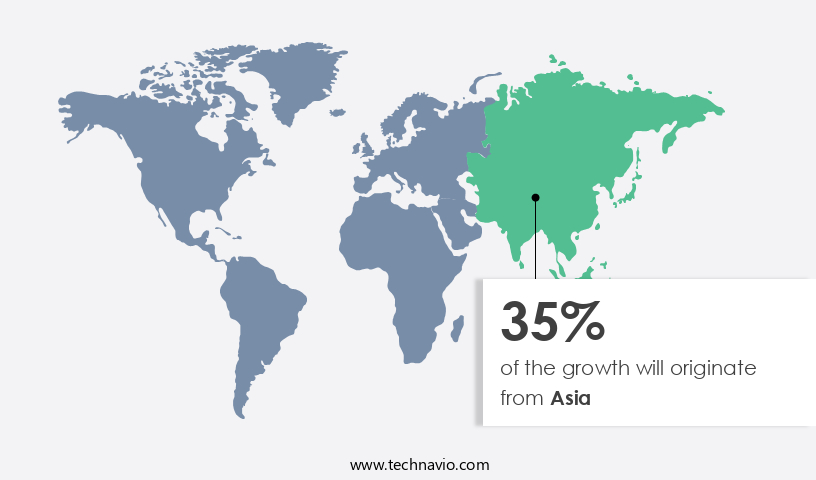
Asia is estimated to contribute 35% to the growth of the global market during the forecast period.Technavio's analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
The market is witnessing significant growth, particularly in North America, due to substantial investments in the pharmaceutical industry, with a focus on oncology. The US, as the major contributor to this region, hosts numerous pharmaceutical giants and has recorded over 1.9 million cancer diagnoses and 609,820 cancer deaths in 2023. Quality control is crucial in the production of liposomal products, ensuring consistency in lipid nanoparticles, liposome size distribution, and surface modification for optimal storage stability and encapsulation efficiency. Oral delivery and injectable formulations, including subcutaneous and intramuscular injections, are popular methods for administering these products. Targeted drug delivery through stealth liposomes and polymeric liposomes enhances bioavailability and reduces side effects.
Veterinary medicine and transdermal delivery are emerging applications, while clinical trials continue to evaluate the potential of liposomal products in various therapeutic areas. Intravenous administration, intravenous drug loading, and controlled and sustained release are other essential aspects of this market. Biocompatible materials and manufacturing processes are key considerations for producing effective and safe liposomal products.
Market Dynamics
Our researchers analyzed the data with 2023 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
What are the key market drivers leading to the rise in the adoption of Liposomal Products Industry?
- The increasing prevalence of fungal disorders serves as the primary catalyst for market growth in this sector.
- Liposomal products have gained significant attention in the healthcare industry due to their effectiveness in treating fungal infections. These products work by either eliminating fungal cells or inhibiting their growth and reproduction. The prevalence of fungal infections is on the rise, particularly among the geriatric population and those with weakened immune systems, including individuals with chronic diseases, HIV, transplant recipients, and long-term consumers of immunosuppressants. If left untreated, fungal infections can lead to chronic conditions. Quality control is crucial in the production of liposomal products to ensure their efficacy and safety. Oral delivery and subcutaneous injection are common methods for administering these products.
- Advanced technologies, such as lipid nanoparticles and targeted drug delivery, enhance the therapeutic potential of liposomal products. Stealth liposomes, which can evade the body's immune system, extend the shelf life of these products. Liposomal technology is not limited to human medicine; it also plays a vital role in veterinary medicine. The growing demand for effective treatments for various animal diseases, coupled with the increasing awareness of the benefits of liposomal products, is expected to drive market growth.
What are the market trends shaping the Liposomal Products Industry?
- The increasing implementation of Quality by Design (QbD) over conventional processes represents a significant market trend. This shift towards QbD signifies a commitment to enhancing product quality and efficiency through proactive design and development strategies.
- The market is experiencing significant growth due to advancements in transdermal delivery and injectable formulations. Liposome size distribution and surface modification are crucial factors influencing the efficacy and stability of these formulations. The QbD (Quality by Design) approach is gaining popularity in the manufacturing of liposomal formulations, as it offers a fact-based, rational approach to decision-making, contrasting traditional trial-and-error methods. Regulatory authorities worldwide are advocating for process and regulatory harmonization, further promoting the adoption of QbD.
- This approach enhances product quality, consistency, and excipient supply, ultimately leading to superior final products or drugs. Liposomal encapsulation efficiency and storage stability are essential considerations in the production of dietary supplements and pharmaceuticals, further driving market growth.
What challenges does the Liposomal Products Industry face during its growth?
- The inadequate reimbursement policies for cancer treatment and liposomal products pose a significant challenge to the industry's growth, as these life-saving therapies often come with high costs that limit access and hinder market expansion.
- The market faces significant challenges due to inadequate reimbursement policies, particularly in the context of cancer treatment. With the increasing number of cancer patients and the growing complexity of diagnosis and treatment, there is a pressing need for affordable healthcare solutions. In developed countries, such as the US, where insurance coverage is widespread, the high out-of-pocket costs of services, high deductibles, premiums, and co-payments for medications pose significant challenges. The lack of adequate information to make informed insurance decisions, long travel distances for care, and limited access to preferred healthcare providers and hospitals further exacerbate these issues. These financial barriers hinder the growth of the market in the region.
- Despite these challenges, advancements in liposome technology, such as drug loading capacity, clinical trials, and the development of polymeric, anionic, and sterically stabilized liposomes, offer promising opportunities for market expansion. The release kinetics of these advanced liposomal formulations are under rigorous investigation to optimize therapeutic efficacy and reduce side effects, making them a valuable asset in the healthcare industry.
Exclusive Customer Landscape
The liposomal products market forecasting report includes the adoption lifecycle of the market, covering from the innovator's stage to the laggard's stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the liposomal products market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape
Key Companies & Market Insights
Companies are implementing various strategies, such as strategic alliances, liposomal products market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
Acthera Therapeutics AG - The company specializes in advanced liposomal technology, utilizing hard-shelled liposomes to deliver bioactive compounds effectively and efficiently, enhancing their absorption and stability.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Acthera Therapeutics AG
- Bausch Lomb Corp.
- Baxter International Inc.
- Galen Ltd.
- GENFIT SA
- Gilead Sciences Inc.
- Innocan Pharma Corp. Ltd.
- Johnson and Johnson Services Inc.
- Leadiant Biosciences Inc.
- Lipella Pharmaceuticals Inc.
- Liposoma B.V.
- Merrimack Pharmaceuticals Inc.
- Nanovex Biotechnologies SL
- Pacira BioSciences Inc.
- Spectrum Pharmaceuticals Inc.
- Taiwan Liposome Co. Ltd.
- Takeda Pharmaceutical Co. Ltd.
- The Lubrizol Corp.
- Vascular Biosciences
- Viatris Inc.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Liposomal Products Market
- In January 2024, Amicus Therapeutics announced the FDA approval of its liposomal Fab Amyloid Binding Agent (FAB), Galafold, for the treatment of Fabry disease. This marked a significant regulatory milestone for the market, expanding the indications for this technology (Amicus Therapeutics Press Release, 2024).
- In March 2024, CuraMedix and LipoScience entered into a strategic partnership to co-develop and commercialize LipoCine, a liposomal ciprofloxacin formulation for the treatment of pediatric patients with cystic fibrosis. This collaboration combined CuraMedix's expertise in liposomal technology with LipoScience's experience in the cystic fibrosis market (CuraMedix Press Release, 2024).
- In May 2024, DSM Nutritional Products completed the acquisition of Martek Biosciences, a leading producer of algae-derived omega-3 lipids. This acquisition significantly expanded DSM's lipid-based nutritional ingredients portfolio and strengthened its position in the market (Bloomberg, 2024).
- In February 2025, Sigma-Tau Pharmaceuticals received European Commission approval for its liposomal formulation of sirolimus, a mTOR inhibitor, for the treatment of lymphangioleiomyomatosis (LAM), a rare lung disease. This approval marked the first European approval for a liposomal sirolimus product and expanded Sigma-Tau's product offerings in the market (Sigma-Tau Pharmaceuticals Press Release, 2025).
Research Analyst Overview
- The market is experiencing significant advancements in drug delivery technology, with a focus on regulatory approvals for various applications. Liposomal creams and patches are gaining traction in pain management and nutrient absorption, while intellectual property protections shape the patent landscape for tumor targeting, vaccine delivery, and gene delivery. In the realm of anti-inflammatory drugs and antiviral therapies, vesicle fusion and membrane permeability enhance the efficacy of liposomal serums. Future trends include bioavailability studies, animal models, and human and in-vivo trials for anti-cancer drugs and wound healing. Competitive advantages lie in optimizing distribution channels, pricing strategies, and cellular uptake for micellar solutions.
- In-vitro assays and in-vivo studies are crucial for understanding the efficacy and safety of these advanced drug delivery systems.
Dive into Technavio's robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Liposomal Products Market insights. See full methodology.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
173 |
|
Base year |
2023 |
|
Historic period |
2018-2022 |
|
Forecast period |
2024-2028 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 10.87% |
|
Market growth 2024-2028 |
USD 3995.5 million |
|
Market structure |
Fragmented |
|
YoY growth 2023-2024(%) |
9.53 |
|
Key countries |
US, Germany, China, France, and Japan |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
What are the Key Data Covered in this Liposomal Products Market Research and Growth Report?
- CAGR of the Liposomal Products industry during the forecast period
- Detailed information on factors that will drive the growth and forecasting between 2024 and 2028
- Precise estimation of the size of the market and its contribution of the industry in focus to the parent market
- Accurate predictions about upcoming growth and trends and changes in consumer behaviour
- Growth of the market across North America, Europe, Asia, and Rest of World (ROW)
- Thorough analysis of the market's competitive landscape and detailed information about companies
- Comprehensive analysis of factors that will challenge the liposomal products market growth of industry companies
We can help! Our analysts can customize this liposomal products market research report to meet your requirements.