Radiofrequency Ablation Devices Market Size 2024-2028
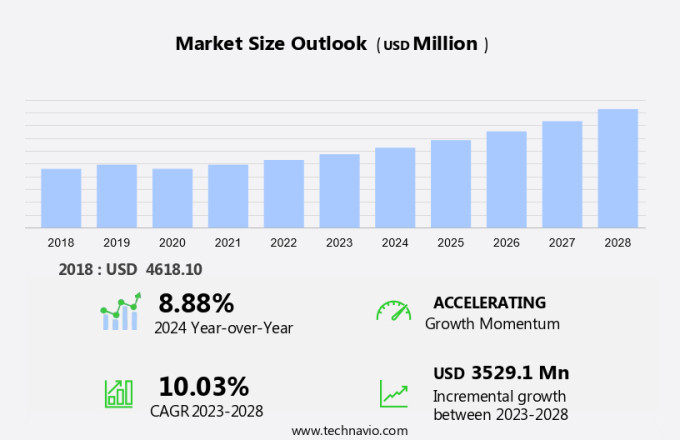
The radiofrequency ablation devices market size is forecast to increase by USD 3.53 billion at a CAGR of 10.03% between 2023 and 2028. The market is experiencing significant growth due to the increasing prevalence of chronic diseases and the expansion of the geriatric population. Chronic pain, particularly in conditions such as cancer and cardiovascular diseases, is a major health concern for this demographic. RFA, a minimally invasive treatment, offers effective pain relief by utilizing high-frequency electrical currents to create controlled lesions in abnormal tissue growths. The high recurrence rate of some ailments post-RFA treatment necessitates continuous innovation in this medical technique. Mergers and acquisitions (M&A) activity in the healthcare industry are also driving market growth. Despite this, the market is expected to continue expanding due to the advantages of RFA over traditional surgical procedures, including fewer complications, shorter hospital stays, and quicker recovery times.
What will be the Size of the Market During the Forecast Period?
The market is witnessing significant growth due to the increasing prevalence of chronic diseases and the growing senior population. These conditions often necessitate elective procedures and surgical therapies, leading to a heightened demand for minimally invasive treatments. RFA devices employ high-frequency electrical currents to create controlled lesions, effectively eliminating abnormal tissue growths. This medical technique has gained popularity in various therapeutic areas, including oncology and atrial fibrillation. The geriatric population, in particular, benefits from RFA procedures due to their minimally invasive nature and reduced recovery time.
Furthermore, the US healthcare expenditure on chronic diseases is projected to increase, fueling the demand for innovative solutions like RFA devices. Strategic initiatives by key players in the market are further driving the growth of the RFA devices market. The market is competitive, with various players offering catheter systems, generators, and accessories. Image-guided techniques are increasingly being integrated into RFA procedures to enhance accuracy and efficiency. The use of RFA devices in cancer care and other chronic diseases is expected to expand, as healthcare systems continue to prioritize minimally invasive treatments. The market is poised for growth, with the potential to revolutionize the way chronic diseases are treated in the US.
Market Segmentation
The market research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2024-2028, as well as historical data from 2018-2022 for the following segments.
- Product
- RF ablation consumables
- RF ablation systems
- RF ablation catheters
- Geography
- North America
- Canada
- US
- Europe
- Denmark
- Asia
- China
- India
- Rest of World (ROW)
- North America
By Product Insights
The RF ablation consumables segment is estimated to witness significant growth during the forecast period. The RF ablation consumables segment holds a significant market share in the global RF ablation devices industry. The demand for RF ablation consumables is increasing due to the high frequency of procedures and their disposable nature. These consumables, which include canulas, needles, single-use probes, single-use electrodes, and RF probes, are essential for performing RF ablation procedures. Since they are meant for one-time use, their recurrent use drives the growth of the global RF ablation consumables market. MRI plays a crucial role in the real-time visualization of RF ablation procedures. Treatment modalities such as microwave ablation and cryoablation are increasingly being used to address chronic diseases like cardiovascular disorders, gynecological disorders, diabetes, and cardiac arrhythmias, including atrial fibrillation.
Furthermore, RF ablation procedures are gaining popularity due to their minimally invasive nature and the ability to provide precise treatment with minimal damage to healthy tissue. The RF ablation consumables market is expected to grow significantly during the forecast period due to the increasing number of RF ablation procedures being performed. The use of RF ablation in treating various chronic diseases is on the rise, and the demand for RF ablation consumables is expected to increase accordingly. The market for RF ablation consumables is expected to be driven by the increasing prevalence of chronic diseases and the growing awareness of minimally invasive treatment modalities.
Get a glance at the market share of various segments Request Free Sample
The RF ablation consumables segment accounted for USD 1.87 billion in 2018 and showed a gradual increase during the forecast period.
Regional Insights
North America is estimated to contribute 45% to the growth of the global market during the forecast period. Technavio's analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
For more insights on the market share of various regions Request Free Sample
In the Americas, the RF ablation devices market is experiencing significant growth due to the rising incidence of chronic diseases such as cancer and cardiovascular diseases (CVDs). According to the Centers for Disease Control and Prevention (CDC), approximately 805,000 Americans experience a heart attack each year, with 605,000 being their first heart attack. Furthermore, RF ablation procedures are commonly used to treat hepatocellular carcinoma (HCC), a significant cause of mortality in Mexico. Consequently, the market for RF ablation devices is expanding in this region. The US and Canada are the primary revenue generators in the North American market due to the high disposable income of the population and advanced healthcare infrastructure.
Our researchers analyzed the data with 2023 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
Market Driver
Increasing prevalence of chronic diseases and growth of geriatric population is the key driver of the market. In the United States, the senior population is experiencing a significant increase in chronic disease prevalence. According to the Centers for Disease Control and Prevention (CDC) and the National Center for Health Statistics (NCHS), approximately 15% of adults in the US had at least two chronic medical conditions in 2021. By contrast, only around 11% of older millennials reported having multiple chronic conditions. The CDC projects that the number of individuals with atrial fibrillation (AF) in the US will reach 12.1 million by 2030. Chronic diseases, including heart disease, stroke, cancer, arthritis, and chronic pain, are becoming more common due to sedentary lifestyles and unhealthy eating habits.
People with chronic pain have pain in various locations. Healthcare systems are responding to this trend by offering elective procedures and surgical therapies, such as Radiofrequency Ablation (RFA), to manage chronic diseases. RFA is an image-guided technique used in cancer care and for treating atrial fibrillation. Companies like AtriCure and Royal Philips are at the forefront of developing innovative RFA devices to address the growing demand for minimally invasive treatments. As the US population ages and the prevalence of chronic diseases continues to rise, the need for advanced medical technologies like RFA devices is expected to increase.
Market Trends
Increasing M and A activity is the upcoming trend in the market. In the healthcare industry, Radiofrequency Ablation Devices have gained significant attention due to their effectiveness in providing pain relief for various conditions, including chronic pain, cancer, and cardiovascular diseases. This medical technique utilizes high-frequency electrical currents to create controlled lesions, eliminating abnormal tissue growths. Ablation procedures are minimally invasive treatments that offer quick patient recovery times. Major players in the market are continuously expanding their product offerings to cater to diverse patient needs. Mergers and acquisitions (M&A) have become a common strategy for companies to enhance their product portfolios.
Affera specializes in producing catheters that generate detailed heart maps, enabling doctors to accurately direct ablation procedures. These catheters apply thermal or electrical energy to scar cardiac tissues, treating conditions like Atrial Fibrillation (AF) and other arrhythmias by blocking abnormal signals within the heart. By incorporating advanced technologies and partnerships, these acquisitions contribute to the growth and differentiation of the companies' offerings, ultimately benefiting patients and healthcare providers.
Market Challenge
High recurrence rate of some ailments post-RFA treatment is a key challenge affecting the market growth. The use of Radiofrequency Ablation (RFA) devices in various medical fields, including oncology, cardiology, and pain management, has been growing. However, the high recurrence rate of RFA treatments may hinder market growth. This issue can discourage patients from undergoing the procedure, leading to a potential decrease in demand for RFA devices. The financial burden of repeated procedures can be significant, particularly for the geriatric population with limited resources. This factor may encourage the exploration of alternative treatment options.
Furthermore, the high recurrence rate can impact research and development efforts in the field of RFA technology. From a healthcare expenditure standpoint, the ongoing costs associated with RFA treatments can be substantial. Catheter systems, generators, and accessories are essential components of RFA procedures, and their repeated use can add to the overall expense. Hospitals and healthcare providers must carefully consider the long-term financial implications of RFA treatments when making purchasing decisions for RFA devices.
Exclusive Customer Landscape
The market forecasting report includes the adoption lifecycle of the market, covering from the innovator's stage to the laggard's stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape
Key Companies & Market Insights
Companies are implementing various strategies, such as strategic alliances, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the market.
Abbott Laboratories - The company offers radiofrequency ablation device named as Simplicity Probe, a self contained, multi electrode radiofrequency, patented SI denervation solution, it features three independent active areas to generally reduce procedure time.
The market research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- AngioDynamics Inc.
- Arthrex Inc.
- AtriCure Inc.
- Avanos Medical Inc.
- BIOTRONIK SE and Co. KG
- Bramsys Industria e Comercio Ltda.
- Conmed Corp.
- Epimed International Inc.
- F Care Systems NV
- Hologic Inc.
- Johnson and Johnson Services Inc.
- Koninklijke Philips N.V.
- Medtronic Plc
- Merit Medical Systems Inc.
- OSYPKA AG
- RF Medical Co. Ltd.
- Smith and Nephew plc
- Stryker Corp.
- Venclose Inc.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key market players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Research Analyst Overview
Radiofrequency ablation (RFA) devices are minimally invasive treatments used in various medical fields, including oncology, cardiology, pain management, and gynecology. These devices utilize high-frequency electrical currents to create controlled lesions in abnormal tissue growths, such as tumors or nerves, targeting specific areas with precision. RFA technology is particularly beneficial for chronic diseases, including cardiovascular disorders like atrial fibrillation and ventricular tachycardia, as well as chronic pain and cancer. These devices employ flexible catheters for precise navigation and manufacturing, often in conjunction with advanced medical imaging techniques such as ultrasound, CT scans, Cannulas, and MRI for real-time visualization. The prevalence of chronic diseases, especially among the geriatric population, has led to increased demand for RFA devices in healthcare systems. Elective procedures using RFA technology offer reduced patient recovery time and lower complications risk compared to traditional surgical therapies.
Furthermore, advanced medical imaging techniques, such as ultrasound, CT scans, and MRI, enable real-time visualization during the procedure, ensuring accurate treatment outcomes. RFA devices consist of generators, catheter systems, and accessories. Companies invest in research and development to miniaturize and make RFA technology more flexible, with the introduction of miniaturized catheters and image-guided techniques. RF generators and consumables are essential components of RFA devices, and their market growth is influenced by the prevalence of chronic diseases, such as Chronic Wound Care, cardiac arrhythmias, atrial fibrillation, and ventricular tachycardia. In addition, the integration of advanced medical imaging and navigation platforms, like the Tissue Engagement Viewer by Royal Philips, enhances the accuracy and efficiency of RFA procedures. RFA technology is used in various treatment modalities, including microwave ablation and cryoablation, and is applied in various fields, such as oncology, cardiology, pain management, gynecology, urology, and cosmetic surgery.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
142 |
|
Base year |
2023 |
|
Historic period |
2018-2022 |
|
Forecast period |
2024-2028 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 10.03% |
|
Market growth 2024-2028 |
USD 3.52 billion |
|
Market structure |
Fragmented |
|
YoY growth 2023-2024(%) |
8.88 |
|
Regional analysis |
North America, Europe, Asia, and Rest of World (ROW) |
|
Performing market contribution |
North America at 45% |
|
Key countries |
US, Denmark, China, Canada, and India |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
|
Key companies profiled |
Abbott Laboratories, AngioDynamics Inc., Arthrex Inc., AtriCure Inc., Avanos Medical Inc., BIOTRONIK SE and Co. KG, Bramsys Industria e Comercio Ltda., Conmed Corp., Epimed International Inc., F Care Systems NV, Hologic Inc., Johnson and Johnson Services Inc., Koninklijke Philips N.V., Medtronic Plc, Merit Medical Systems Inc., OSYPKA AG, RF Medical Co. Ltd., Smith and Nephew plc, Stryker Corp., and Venclose Inc. |
|
Market dynamics |
Parent market analysis, market growth inducers and obstacles, market forecast, fast-growing and slow-growing segment analysis, COVID-19 impact and recovery analysis and future consumer dynamics, market condition analysis for the forecast period |
|
Customization purview |
If our market report has not included the data that you are looking for, you can reach out to our analysts and get segments customized. |
What are the Key Data Covered in this Market Research and Growth Report?
- CAGR of the market during the forecast period
- Detailed information on factors that will drive the market growth and forecasting between 2024 and 2028
- Precise estimation of the size of the market and its contribution of the market in focus to the parent market
- Accurate predictions about upcoming market growth and trends and changes in consumer behaviour
- Growth of the market across North America, Europe, Asia, and Rest of World (ROW)
- Thorough analysis of the market's competitive landscape and detailed information about companies
- Comprehensive analysis of factors that will challenge the growth of market companies
We can help! Our analysts can customize this market research report to meet your requirements. Get in touch