APAC Coronavirus Test Kits Market Size 2024-2028
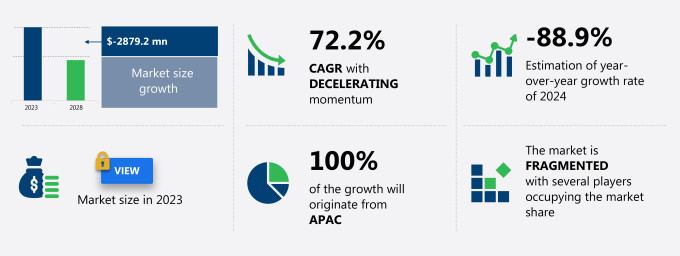
The Asia-Pacific (APAC) - Coronavirus test kits market size is estimated to decrease by USD 2.88 billion and decline at a CAGR of 72.2% between 2023 and 2028. The coronavirus test kits market is experiencing significant growth due to several key factors. In the Asia-Pacific region, the rise in the availability of various types of test kits is driving market expansion. Advanced technology is also playing a crucial role in the development of rapid and accurate test kits, which is a major trend in the market. However, challenges persist, including the shortage of testing kits and lack of laboratory infrastructure in developing countries, which hinder market growth. Despite these challenges, the market is expected to continue its upward trajectory as the demand for coronavirus tests remains high. The need for efficient and reliable testing solutions to contain the spread of the virus is a pressing requirement, making the coronavirus test kits market an essential sector to watch.
APAC Coronavirus Test Kits Market Overview
To know more about APAC Coronavirus Test Kits Market size Download Report Sample
Market Dynamics
The coronavirus test kits market has experienced significant growth due to the ongoing global health crisis. Antigen rapid tests have gained popularity for their quick results, making them essential tools in breaking the infection chain. From a public health perspective, regular testing frequency is crucial in reducing the effective reproduction number and ultimately, the number of deaths and intensive care unit (ICU) admissions. From a health economics standpoint, the health benefits of early diagnosis through coronavirus test kits outweigh the testing costs. False negatives and positives are important considerations, with sensitivity analysis used to assess test accuracy. Risk factors, such as age and underlying health conditions, influence the infection fatality rates and the importance of testing from a consumer perspective. Epidemiological data plays a vital role in determining testing strategies, with mass testing essential for controlling outbreaks. Personal protective measures should always be employed during specimen collection to minimize the risk of infection. Serological tests, which detect antibodies, provide valuable information on past infections and immunity. Incorporating health benefits and infection fatality rates into quality-adjusted life year (QALY) analysis can help inform decision-making in the use of coronavirus test kits. Vaccination, while a crucial long-term solution, does not replace the importance of accurate and timely testing.
Key Market Driver
The rise in the availability of various types of test kits in APAC is notably driving market growth. The coronavirus test kits market in the APAC region has experienced substantial growth, with an array of test types becoming increasingly accessible. Among these, RT-PCR test kits have gained significant popularity due to their ability to detect the presence of the virus by amplifying its genetic material. This gold standard diagnostic method is extensively used in the region, particularly in countries like South Korea and Singapore, which have successfully employed mass testing to identify and isolate infected individuals, thereby controlling the infection chain. From a public health perspective, the effectiveness of testing is measured by the infection fatality rate, the number of deaths per infection, and the effective reproduction number, which represents the average number of secondary infections caused by a single infected person. False negatives and positives are significant concerns in testing, and the sensitivity analysis of various test types plays a crucial role in determining their accuracy. Besides RT-PCR tests, antigen rapid tests and serological tests, which detect the presence of antigens and antibodies, respectively, are also gaining traction in the market. Swab tests and blood tests are the most common sample types for these tests.
Further, the regulatory framework for coronavirus test kits is stringent, ensuring their quality and reliability. The healthcare organizations and hospitals rely on these tests to make informed decisions regarding clinical events, such as admissions to intensive care units, and the implementation of personal protective measures. The health economics of testing, including testing frequency, costs, and health benefits, are essential considerations for both public health authorities and consumers. Molecular testing, such as RT-PCR, and serological testing offer distinct advantages and challenges, and the choice of test type depends on various factors, including the stage of the epidemic, the availability of resources, and the specific clinical scenario. Epidemiological data is essential to inform testing strategies and policies, and ongoing research continues to improve the accuracy and accessibility of coronavirus test kits. Thus, such factors are driving the growth of the market during the forecast period.
Significant Market Trends
The advancements in technology to develop rapid and accurate test kits is the key trend in the market. The coronavirus test kits market in the Asia Pacific (APAC) region has experienced substantial technological advancements, particularly in the production of swift and precise test kits for the identification of the coronavirus. These innovations have significantly transformed the coronavirus test kits industry in APAC, offering enhanced efficiency and accuracy in diagnosing the virus. One of the significant technological breakthroughs in the coronavirus test kits market in APAC is the emergence of rapid antigen test kits. These test kits identify specific proteins on the coronavirus surface and can be used with nasal or throat swab samples, delivering results in a matter of minutes.
In contrast, traditional molecular tests, such as PCR, necessitate laboratory processing and take longer to yield results. The utilization of these test kits from a consumer perspective is crucial in interrupting the infection chain, reducing the number of deaths and intensive care unit admissions, and contributing to public health. The effectiveness of testing frequency in controlling the spread of the virus is contingent upon the sensitivity and specificity of the test kits, which is where the role of quality-adjusted life years (QALYs) and health economics comes into play. The inaccuracy of test results, including false negatives and positives, necessitates the implementation of personal protective measures and mass testing. Epidemiological data, infection fatality rates, clinical events, and diagnosis are all factors that influence the health benefits and testing costs. Sensitivity analysis is essential to evaluate the impact of various test types, such as molecular testing and serological testing, and sample types, including swab tests and blood tests, on the overall market dynamics. The regulatory frameworks for the approval and distribution of coronavirus test kits vary among countries in the APAC region, and healthcare organizations play a crucial role in ensuring the availability and accessibility of these test kits. Thus, such trends will shape the growth of the market during the forecast period.
Major Market Challenge
The shortage of testing kits and lack of laboratory infrastructure in developing countries is the major challenge that affects the growth of the market. The ongoing coronavirus pandemic has led to a growth in demand for COVID-19 testing kits, particularly in APAC countries experiencing a peak in infection rates. India, Australia, and Japan are among the nations grappling with testing kit shortages due to an increase in positive cases and the fear of community transmission. Diagnostic companies are struggling to meet the demand, exacerbated by travel restrictions and import bans on test kits from abroad. From a public health perspective, timely and accurate diagnosis is crucial in breaking the infection chain and reducing the effective reproduction number. The use of antigen rapid tests and molecular testing, including swab tests and blood tests, plays a significant role in diagnosis.
However, the accuracy of these tests, including false negatives and positives, must be considered in the context of healthcare economics and the potential health benefits and costs. Quality-adjusted life years, infection fatality rates, clinical events, and sensitivity analysis are essential factors in evaluating the health benefits of testing. The regulatory framework for coronavirus test kits is also critical in ensuring their effectiveness and safety. Consumer perspective, testing frequency, and personal protective measures are also essential considerations in the mass testing strategy. Serological tests, which detect antibodies, can provide valuable epidemiological data and complement molecular testing in understanding the spread of the virus. Ultimately, the use of various test types and sample types, along with effective public health measures, can help mitigate the impact of the pandemic. Hence, the above factors will impede the growth of the market during the forecast period.
Market Segment
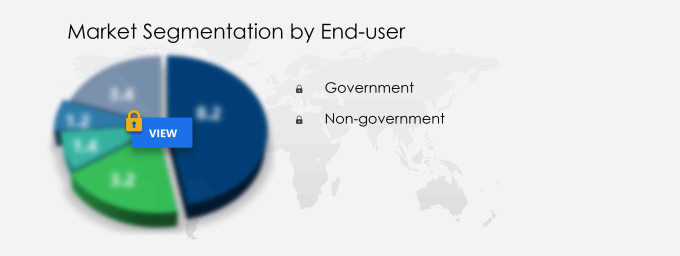
End-user
The government segment is estimated to witness significant growth during the forecast period. The Coronavirus Test Kits Market in the Asia-Pacific (APAC) region is significantly influenced by the government segment, which holds the largest revenue share. This is primarily due to the increased investments by government organizations in the development of new Antigen rapid tests and the acceleration of COVID-19 testing initiatives. The unmet testing needs in APAC countries, such as Singapore, China, South Korea, and India, are being addressed through government support. Regulatory authorities in the region have eased compliance by granting provisional authorization for new launches, enabling the rapid entry of improved testing kits.
Get a glance at the market segment contribution View the PDF Sample
From a public health perspective, effective diagnosis is crucial in managing the infection chain, reducing the number of deaths, and minimizing the need for intensive care unit (ICU) treatment. The consumer perspective focuses the importance of testing frequency, accuracy, and health benefits, which are influenced by factors such as infection fatality rates, clinical events, and sensitivity analysis. The testing costs, false negatives/positives, and personal protective measures also impact the market dynamics. The availability of various test types, including molecular testing and serological testing, and sample types, such as swab tests and blood tests, cater to diverse healthcare organization requirements. The regulatory framework and inaccuracy issues, including false-positives and false-negatives, are key challenges for market growth. Health economics, including vaccination and health benefits, are also significant factors influencing the market. Epidemiological data plays a vital role in understanding the infection spread and informing testing strategies. Overall, the Coronavirus Test Kits Market in APAC is driven by the need for effective and timely diagnosis, regulatory support, and the evolving healthcare landscape.
Who are the Major APAC Coronavirus Test Kits Market Companies?
Companies are implementing various strategies, such as strategic alliances, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the market.
- 3D Biomedicine Science and Technology Co. Ltd.: The company offers coronavirus test kits such as ANDiS SARS -Cov-2 RT-qPCR Detection Kit, ANDiS SARS -Cov-2 and Influenza A B RT-qPCR Detection Kit, and ANDis 350 automated nucleic acid extraction system.
The research report also includes detailed analyses of the competitive landscape of the market and information about 20 market companies, including:
- Abbott Laboratories
- altona Diagnostics GmbH
- BGI Group
- Bio Rad Laboratories Inc.
- CAMTECH DIAGNOSTICS
- Co Diagnostics Inc.
- Danaher Corp.
- Denka Co. Ltd.
- Edinburgh Genetics Ltd.
- F. Hoffmann La Roche Ltd.
- Genes2Me Pvt. Ltd.
- Guangzhou Wondfo Biotech Co. Ltd.
- Hologic Inc.
- JN Medsys Pte. Ltd.
- KogeneBiotech
- Kurabo Industries Ltd.
- Maccura Biotechnology Co. Ltd.
- Mylab Discovery Solutions Pvt Ltd.
- QIAGEN NV
- Sd Biosensor Inc.
- Seegene Inc.
- SolGent Co. Ltd.
- Sysmex Corp.
- Tata Sons Pvt. Ltd.
- Thermo Fisher Scientific Inc.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key market players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Segment Overview
The market research report provides comprehensive data (region wise segment analysis), with forecasts and estimates in "USD Billion" for the period 2024 to 2028 for the following segments
- End-user Outlook
- Government
- Non-government
- Type Outlook
- Rapid test kit
- RT-PCR
- Others
Market Analyst Overview
The coronavirus test kits market is witnessing significant growth due to the ongoing global health crisis. Antigen rapid tests have gained popularity for their quick results, making mass testing a viable solution to break the infection chain. From a public health perspective, effective testing frequency is crucial in reducing the effective reproduction number and minimizing the spread of the virus. The health benefits of timely diagnosis extend beyond individual health, as it also contributes to reducing the burden on healthcare organizations, particularly in terms of intensive care unit (ICU) admissions and deaths. However, health economics plays a vital role in determining testing costs and the consumer perspective. False negatives and positives are concerns that require sensitivity analysis to ensure accurate clinical events and infection fatality rates. Regulatory frameworks are essential in ensuring the quality of coronavirus test kits, with both molecular testing and serological testing having their unique advantages and challenges. Specimen collection methods, such as swab tests and blood tests, also impact the accuracy of test results. The use of personal protective measures is crucial during specimen collection and testing processes. Vaccination and ongoing research for better treatments continue to be critical components in managing the pandemic. Epidemiological data and infection chain analysis remain essential in guiding testing strategies and informing public health policies. The market dynamics of coronavirus test kits are influenced by various factors, including testing costs, regulatory frameworks, and the ongoing development of new test types.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
162 |
|
Base year |
2023 |
|
Forecast period |
2024-2028 |
|
Growth momentum & CAGR |
Decelerate at a CAGR of -72.2% |
|
Market growth 2024-2028 |
USD -2.88 billion |
|
Market structure |
Fragmented |
|
YoY growth 2023-2024(%) |
-88.9 |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
|
Key companies profiled |
3D Biomedicine Science and Technology Co. Ltd., Abbott Laboratories, altona Diagnostics GmbH, BGI Group, Bio Rad Laboratories Inc., CAMTECH DIAGNOSTICS, Co Diagnostics Inc, Danaher Corp., Denka Co. Ltd., Edinburgh Genetics Ltd., F. Hoffmann La Roche Ltd., Genes2Me Pvt. Ltd., Guangzhou Wondfo Biotech Co. Ltd., Hologic Inc., JN Medsys Pte. Ltd., KogeneBiotech, Kurabo Industries Ltd., Maccura Biotechnology Co. Ltd, Mylab Discovery Solutions Pvt Ltd., QIAGEN N.V., Sd Biosensor Inc., Seegene Inc., SolGent Co. Ltd., Sysmex Corp., Tata Sons Pvt. Ltd., and Thermo Fisher Scientific Inc. |
|
Market dynamics |
Parent market analysis, market forecasting, Market growth inducers and obstacles, Fast-growing and slow-growing segment analysis, Market growth and Forecasting, COVID 19 impact and recovery analysis and future consumer dynamics, Market condition analysis for market forecast period |
|
Customization purview |
If our market report has not included the data that you are looking for, you can reach out to our analysts and get segments customized. |
What are the Key Data Covered in this APAC Coronavirus Test Kits Market Research Report?
- CAGR of the market during the forecast period
- Detailed information on factors that will drive the market growth and forecasting of the market between 2024 and 2028
- Precise estimation of the market size and its contribution to the parent market
- Accurate predictions about upcoming market trends and analysis and changes in consumer behavior
- Growth of the market across APAC
- Thorough market growth analysis of the market's competitive landscape and detailed information about companies
- Comprehensive market analysis and report on the factors that will challenge the market research and growth of market companies
We can help! Our analysts can customize this market research report to meet your requirements.