Bioprocess Validation Market Size 2024-2028
The bioprocess validation market size is forecast to increase by USD 364 billion at a CAGR of 12.88% between 2023 and 2028.
- The market is witnessing significant growth due to the increasing demand for biopharmaceuticals and the adoption of single-use technologies. Biopharmaceuticals are gaining popularity In the healthcare industry due to their ability to treat complex diseases, leading to a surge in demand for their production. Single-use technologies, which offer advantages such as reduced costs, improved product quality, and increased efficiency, are increasingly being adopted for bioprocess validation. However, the high costs associated with bioprocess validation remain a challenge for market growth. Bioprocess tecnology is a critical step in ensuring the safety and efficacy of biopharmaceuticals, making it essential for market players to invest in advanced technologies and techniques to streamline the validation process and reduce costs.The market is expected to continue its growth trajectory In the coming years, driven by these trends and the increasing focus on developing innovative biopharmaceutical products.
What will be the Size of the Bioprocess Validation Market During the Forecast Period?
- The market encompasses the technologies and services employed to ensure the production of high-quality biopharmaceuticals, including impurities testing for vaccines, drug products, monoclonal antibodies, recombinant proteins, and biosimilars. With the ongoing development of precision medicines and vaccines for chronic diseases, such as the SARS-CoV-2 virus, the market's significance continues to grow. The market consists of various segments, including in-house and outsourcing services, with leading biopharmaceutical companies increasingly relying on outsourcing to manage bioproduction activities. The biopharmaceutical manufacturing sector's expansion is driven by socioeconomic factors, increasing demand for biologic generic drugs, and the need for compatibility, microbiological, physiochemical, and integrity testing services.
- Key components of bioprocess validation include filter elements, mixing systems, and other critical equipment used throughout the bioproduction process. The market's trends include the increasing use of advanced technologies for bioprocess validation, such as automation, artificial intelligence, and machine learning, to improve efficiency and accuracy.
How is this Bioprocess Validation Industry segmented and which is the largest segment?
The bioprocess validation industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2024-2028, as well as historical data from 2018-2022 for the following segments.
- End-user
- Pharmaceutical companies
- Contract development and manufacturing organizations
- Others
- Type
- In-house
- Outsourced
- Geography
- North America
- Canada
- US
- Europe
- Germany
- Asia
- China
- Japan
- Rest of World (ROW)
- North America
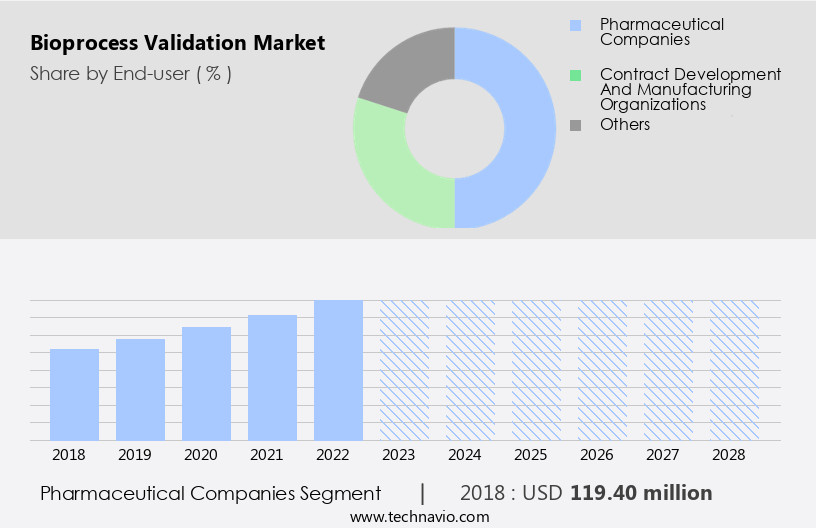
By End-user Insights
- The pharmaceutical companies segment is estimated to witness significant growth during the forecast period.
The market encompasses the validation of biopharmaceutical manufacturing processes for various pharmaceutical companies, including large enterprises and SMEs. Large companies, such as Pfizer, J and J, and Novartis, contribute significantly to the market due to their extensive resources, expertise, and adherence to industry best practices. They invest heavily in research and development (RD) expenditure for the production of complex biologics, including vaccines for SARS-CoV-2, monoclonal antibodies, recombinant proteins, and biosimilars. The market includes several segments, including impurities testing, vaccines, drug products, and biosimilars. Validation procedures involve analytical testing methods, cleaning procedures, and compliance with regulatory standards for drug safety. The market also includes services for precision medicines, cell therapy, and gene therapy.
Contract service providers offer digital tools, continuous process monitoring, real-time release testing, advanced analytics, and modelling techniques. The biopharmaceutical manufacturing sector is driven by the increasing demand for biologic drugs and bioproduction volumes. Automation technologies, including robotics and single-use systems, are also transforming the industry. Socioeconomic factors, such as chronic diseases and aging populations, further fuel market growth. The market includes services for extractable testing, microbiological testing, physiochemical testing, and compatibility testing, as well as bioprocess instruments, such as bioreactors, chromatography systems, and filtration elements.
Explore Bioprocess Validation Industry Segments Request Free Sample
The Pharmaceutical companies segment was valued at USD 119.40 billion in 2018 and showed a gradual increase during the forecast period.
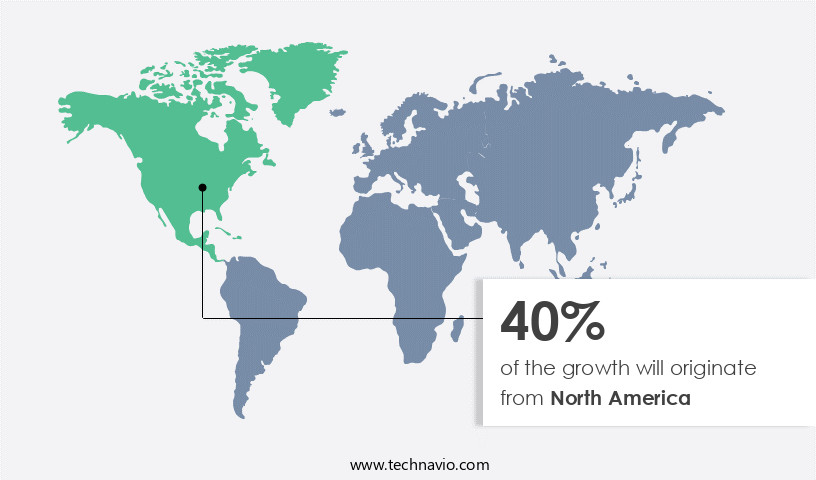
Regional Analysis
- North America is estimated to contribute 40% to the growth of the global market during the forecast period.
Technavio’s analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
For more insights on the market share of various regions, Request Free Sample
The market in North America is experiencing growth due to the increasing demand for biopharmaceuticals and the importance of ensuring product safety. Key industry players, including Pfizer Inc, Merck Group, Biogen Inc, and Amgen Inc, are based In the region and are investing in bioprocess validation to maintaIn the quality and safety of their biologic drugs. Merck Group employs a risk-based approach to bioprocess validation, optimizing validation procedures and protocols to maximize efficiency. The market is further driven by the expanding Biotechnology industry, with significant investments In the Biopharmaceutical manufacturing sector. This includes the development of advanced analytics, modeling techniques, and automation technologies, such as bioreactors, chromatography systems, and monoclonal antibodies.
The market encompasses various testing services, including Microbiological Testing Services, Integrity Testing Services, and Physiochemical Testing Services, as well as Extractable testing services and Cleaning procedures. The market's growth is influenced by the increasing volumes of bioproduction activities, biologics market expansion, and the Biosimilars market. Socioeconomic factors and regulatory requirements also play a role in market growth. Bioprocess instruments, such as high-resolution mass spectrometry, are utilized for the detection of degradation products and impurities. The market is further segmented into the In-house and Contract service provider segments. Digital tools, including Continuous process monitoring, Real-time release testing, and Advanced analytics, are also integral to the market.
The Bioprocessing facilities are subjected to rigorous validation procedures and protocols, ensuring the production of safe and effective biologic drugs for the treatment of chronic diseases, such as cancer and neurological disorders.
Market Dynamics
Our researchers analyzed the data with 2023 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
What are the key market drivers leading to the rise In the adoption of Bioprocess Validation Industry?
Increasing demand for biopharmaceuticals is the key driver of the market.
- Bioprocess validation plays a crucial role In the biopharmaceutical manufacturing process, ensuring the production of consistent and high-quality drug products, including vaccines and monoclonal antibodies. Impurities, such as degradation products and residuals, must be rigorously tested using precision analytical methods, including microbiological, physiochemical, and extractable testing services. The validation procedures and protocols are essential for drug safety and are particularly important In the production of biosimilars, which must demonstrate comparable quality to their reference products. The biotechnology industry's increasing focus on biologics, including monoclonal antibodies and recombinant proteins, for the treatment of chronic diseases, has led to a significant increase in bioprocess validation activities.
- The biopharmaceutical manufacturing sector's outsourcing services have also grown, with contract service providers offering expertise in bioprocess validation and the use of advanced digital tools, such as continuous process monitoring, real-time release testing, and advanced analytics. Biopharmaceutical RD expenditure continues to rise, driven by the growing demand for biologic drugs and the development of new technologies, such as cell therapy and gene therapy. Automation technologies, including bioreactors, chromatography systems, and single-use systems, are increasingly being adopted to improve efficiency and reduce costs. In-house bioprocess validation teams and contract service providers must ensure that these advanced technologies are validated to meet regulatory requirements and maintain product quality and consistency.
- Bioprocess validation is also essential In the production of vaccines for emerging diseases, such as SARS-CoV-2 virus. The validation of the manufacturing process for these vaccines must be rigorous to ensure their safety and efficacy. The validation of the cleaning procedures, filter elements, and mixing systems is particularly important to prevent contamination and maintain product purity. In summary, bioprocess validation is a critical component of the biopharmaceutical manufacturing process, ensuring the production of safe and effective drug products. The increasing demand for biologics, the adoption of advanced technologies, and the development of new therapies require a robust validation approach to maintain product quality and consistency. The use of trained professionals, digital tools, and outsourcing services can help meet these challenges and ensure regulatory compliance.
What are the market trends shaping the Bioprocess Validation Industry?
Increasing adoption of single-use technologies is the upcoming market trend.
- Bioprocess validation plays a crucial role in ensuring the quality and safety of drug products, including vaccines and monoclonal antibodies, In the biopharmaceutical manufacturing process. Impurities, such as degradation products and residuals, must be rigorously tested using analytical methods, including microbiological, physiochemical, and extractable testing services. Bioprocess instruments, such as bioreactors and chromatography systems, are essential for bioprocess validation. The adoption of single-use systems (SUTs) in bioprocessing has gained momentum due to their ease of implementation for bioprocess validation. SUTs eliminate the need for cleaning and sterilization procedures, saving time and resources. This is particularly advantageous for smaller biotech companies.
- In the context of the current global health crisis, SUTs have been instrumental In the production of vaccines for the SARS-CoV-2 virus. Bioprocess validation also plays a significant role In the development and production of precision medicines, biosimilars, and cell and gene therapies. Validation procedures and protocols are essential for ensuring the integrity of these complex biologic drugs. Trained professionals conduct these procedures using advanced analytics, modeling techniques, and digital tools for continuous process monitoring and real-time release testing. The biopharmaceutical manufacturing sector's bioproduction activities are subject to stringent regulations, and bioprocess validation is a critical component of maintaining drug safety.
- The biotechnology industry's investment in RD expenditure continues to grow, leading to an increase in bioprocessing facilities and volumes. Automation technologies, such as robotics and automation systems, are increasingly being used to streamline bioprocess validation processes. In summary, bioprocess validation is a vital aspect of the biopharmaceutical manufacturing process, ensuring the quality, safety, and integrity of drug products, including vaccines, monoclonal antibodies, and biosimilars. The adoption of single-use systems and advanced technologies, such as automation and digital tools, is driving innovation and efficiency in bioprocess validation.
What challenges does the Bioprocess Validation Industry face during its growth?
High costs associated with bioprocess validation is a key challenge affecting the industry growth.
- Bioprocess validation is an essential component of the biopharmaceutical manufacturing process, ensuring the production of safe and effective drug products, including vaccines, monoclonal antibodies, recombinant proteins, and biosimilars. Impurities, such as degradation products, extractables, and leachables, must be rigorously tested using precision analytical methods, including microbiological, physiochemical, and compatibility testing services. The biotechnology industry's increasing focus on developing treatments for chronic diseases, precision medicines, cell therapy, and gene therapy, has led to a significant increase in bioprocess validation expenditure. The biopharmaceutical manufacturing sector's outsourcing services, including contract service providers and digital tools, have also contributed to the market's growth.
- Bioprocess validation procedures and protocols require trained professionals to execute and oversee the validation process. Bioprocess instruments, such as bioreactors, chromatography systems, and filtration elements, are essential for the validation process. The increasing adoption of automation technologies, robotics, and single-use systems has streamlined the bioprocessing facilities' operations. The residuals testing segment, which includes cleaning procedures and real-time release testing, is a significant contributor to the market. Advanced analytics, modeling techniques, and continuous process monitoring have also gained popularity In the industry. The biopharmaceutical RD expenditure, driven by leading biopharmaceutical companies, has fueled the demand for bioprocess validation services. Socioeconomic factors, such as the increasing prevalence of chronic diseases and the growing biosimilars and biologics market, have further boosted the market's growth.
Exclusive Customer Landscape
The bioprocess validation market forecasting report includes the adoption lifecycle of the market, covering from the innovator’s stage to the laggard’s stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the bioprocess validation market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape
Key Companies & Market Insights
Companies are implementing various strategies, such as strategic alliances, bioprocess validation market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence In the industry.
Agilent Technologies Inc. - The company provides bioprocess validation services through its ClinGuide GMP CRISPR sgRNAs for Human Therapeutics system. This system ensures compliance with Good Manufacturing Practices (GMP) In the production of CRISPR synthetic gene-editing tools for human therapeutics.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Agilent Technologies Inc.
- Asahi Kasei Corp.
- Avantor Inc.
- Bangalore Biotech labs Pvt. Ltd.
- Charles River Laboratories International Inc.
- Corning Inc.
- Danaher Corp.
- Eurofins Scientific SE
- GEA Group AG
- Hangzhou Cobetter Filtration Equipment Co. Ltd.
- Laboratory Corp. of America Holdings
- Lonza Group Ltd.
- Meissner Filtration Products Inc.
- Merck KGaA
- Porvair Plc
- ProPharma Group Holdings LLC
- Sartorius AG
- SGS SA
- Thermo Fisher Scientific Inc.
- W. L. Gore and Associates Inc.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Research Analyst Overview
The market encompasses a range of services and technologies that ensure the consistency and quality of biopharmaceutical manufacturing processes. These processes involve the production of complex drug products, such as monoclonal antibodies, recombinant proteins, and cell and gene therapies, which are used to treat chronic diseases and address various health conditions. Bioprocess validation plays a critical role in maintaining the integrity of these manufacturing processes, which can be complex and involve multiple stages. Validation procedures and protocols are employed to ensure that the processes are functioning optimally and consistently, from raw material sourcing to final product release. Trained professionals In the biotechnology industry oversee these validation activities, which may include analytical testing methods, cleaning procedures, and the use of advanced digital tools for continuous process monitoring and real-time release testing.
These tools enable the detection of degradation products and other impurities, ensuring the safety and efficacy of the final drug product. Biopharmaceutical manufacturing processes can be outsourced to contract service providers, who offer expertise in bioprocessing facilities and the use of specialized bioprocess instruments, such as bioreactors, chromatography systems, and filter elements. Automation technologies, including robotics and single-use systems, are also utilized to enhance efficiency and reduce the risk of contamination. The market is driven by the increasing demand for biologic drugs, which account for a significant portion of the global pharmaceutical market. The biologics market is expected to grow steadily In the coming years due to the rising prevalence of chronic diseases and the development of precision medicines and biosimilars.
The market for bioprocess validation services is further fueled by the need for regulatory compliance and the increasing focus on ensuring drug safety. Validation procedures and protocols are essential for demonstrating compliance with regulatory guidelines and ensuring the quality and consistency of biopharmaceutical manufacturing processes. The use of advanced analytics, modeling techniques, and in silico modeling is also driving innovation In the market. These technologies enable the prediction of process performance and the optimization of manufacturing conditions, leading to improved product quality and reduced production costs. Socioeconomic factors, such as increasing healthcare spending and the growing demand for affordable healthcare solutions, are also contributing to the growth of the market.The market is expected to continue expanding In the coming years, driven by the increasing demand for biologic drugs and the need for regulatory compliance and quality assurance In the biopharmaceutical manufacturing sector.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
168 |
|
Base year |
2023 |
|
Historic period |
2018-2022 |
|
Forecast period |
2024-2028 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 12.88% |
|
Market growth 2024-2028 |
USD 364 million |
|
Market structure |
Fragmented |
|
YoY growth 2023-2024(%) |
11.55 |
|
Key countries |
US, Germany, China, Canada, and Japan |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
What are the Key Data Covered in this Bioprocess Validation Market Research and Growth Report?
- CAGR of the Bioprocess Validation industry during the forecast period
- Detailed information on factors that will drive the growth and forecasting between 2024 and 2028
- Precise estimation of the size of the market and its contribution of the industry in focus to the parent market
- Accurate predictions about upcoming growth and trends and changes in consumer behaviour
- Growth of the market across North America, Europe, Asia, and Rest of World (ROW)
- Thorough analysis of the market’s competitive landscape and detailed information about companies
- Comprehensive analysis of factors that will challenge the bioprocess validation market growth of industry companies
We can help! Our analysts can customize this bioprocess validation market research report to meet your requirements.