Effervescent Tablet Market Size 2025-2029
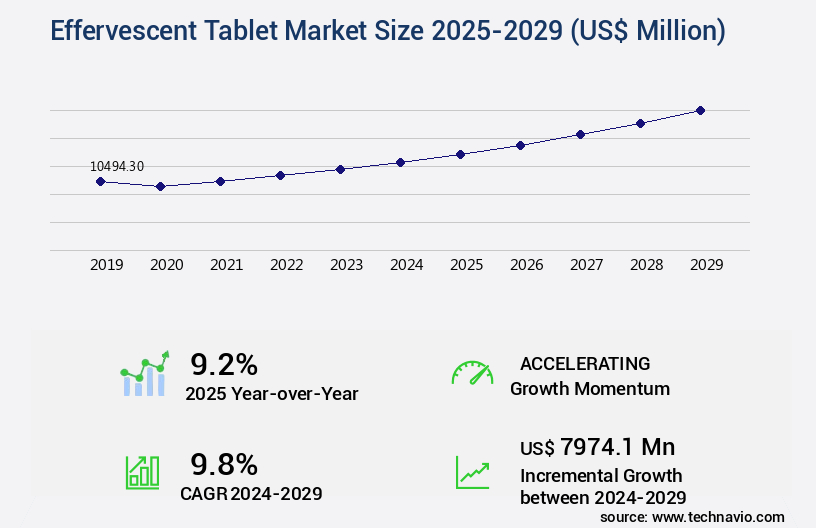
The effervescent tablet market size is valued to increase USD 7.97 billion, at a CAGR of 9.8% from 2024 to 2029. Growing prevalence of chronic diseases will drive the effervescent tablet market.
Major Market Trends & Insights
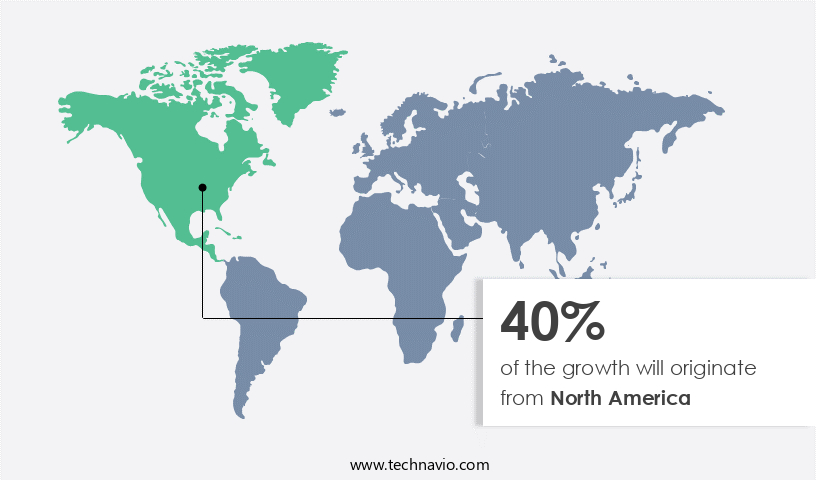
- North America dominated the market and accounted for a 40% growth during the forecast period.
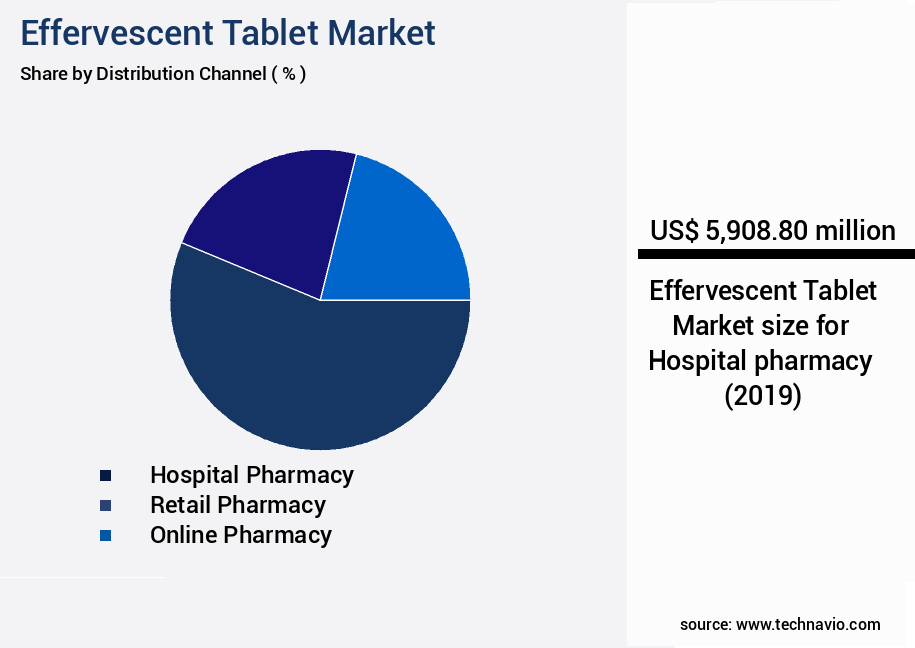
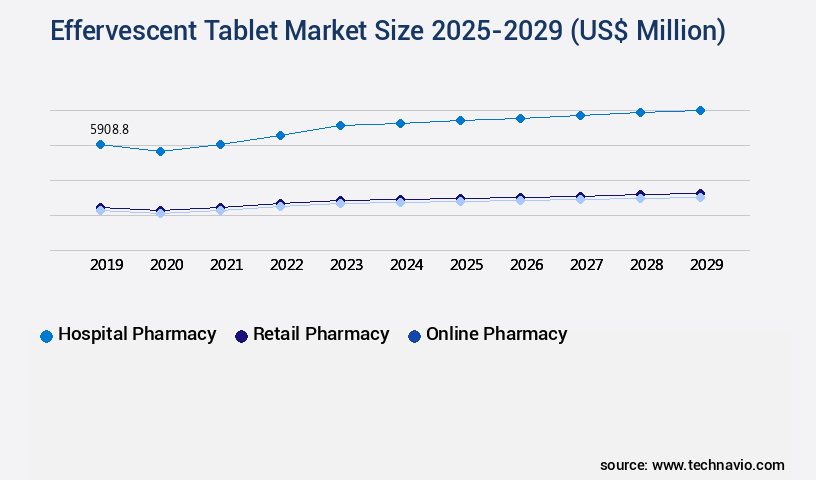
- By Distribution Channel - Hospital pharmacy segment was valued at USD 5.91 billion in 2023
- By Type - Prescription segment accounted for the largest market revenue share in 2023
Market Size & Forecast
- Market Opportunities: USD 103.84 million
- Market Future Opportunities: USD 7974.10 million
- CAGR : 9.8%
- North America: Largest market in 2023
Market Summary
- The market represents a dynamic and continually evolving landscape, driven by advancements in core technologies and applications. These tablets, which release their contents when dissolved in water, offer enhanced bioavailability and patient convenience. One significant driver of market growth is the rising prevalence of chronic diseases, necessitating more effective and accessible medication delivery systems. According to a report, the organic effervescent tablets segment is expected to witness a substantial growth rate due to increasing consumer preference for natural and additive-free products.
- However, the market is not without challenges. Stringent regulations and guidelines related to medicines pose significant hurdles, necessitating compliance and adherence to rigorous quality standards. Despite these obstacles, opportunities abound, particularly in emerging markets and for specialized applications. For instance, the market share of effervescent tablets in the pediatric segment is projected to expand due to their ease of administration and palatability.
What will be the Size of the Effervescent Tablet Market during the forecast period?
Get Key Insights on Market Forecast (PDF) Request Free Sample
How is the Effervescent Tablet Market Segmented and what are the key trends of market segmentation?
The effervescent tablet industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2025-2029, as well as historical data from 2019-2023 for the following segments.
- Distribution Channel
- Hospital pharmacy
- Retail pharmacy
- Online pharmacy
- Type
- Prescription
- Over-the-counter (OTC)
- Application
- Pharmaceuticals
- Dietary supplements
- Dental products
- Others
- Formulation Type
- Dry Granulation
- Wet Granulation
- Direct Compression
- End-User
- Adults
- Pediatric
- Geriatric
- Geography
- North America
- US
- Canada
- Europe
- France
- Germany
- Italy
- UK
- Middle East and Africa
- Egypt
- KSA
- Oman
- UAE
- APAC
- China
- India
- Japan
- South America
- Argentina
- Brazil
- Rest of World (ROW)
- North America
By Distribution Channel Insights
The hospital pharmacy segment is estimated to witness significant growth during the forecast period.
The market is experiencing significant growth due to various factors, primarily driven by the increasing prevalence of diseases such as flu and gastrointestinal disorders. This trend is particularly noticeable in the hospital pharmacy segment, which is expected to expand substantially during the forecast period. Large hospitals and clinics, equipped with advanced medical infrastructure, procure high-quality medical products and consumables in bulk, thereby fueling the demand for effervescent tablets. Furthermore, the rising number of admissions of patients with symptoms like headache, nausea, vomiting, photophobia, and phonophobia will continue to boost the market's growth. The effervescent tablets' ability to provide quick relief and ease of administration is a significant factor contributing to their popularity.
Additionally, the formulation and manufacturing processes for effervescent tablets are continually evolving, with advancements in dissolution testing, powder flow properties, particle size distribution, water absorption rate, density measurement, coating thickness uniformity, chemical stability, taste masking techniques, physical stability, shelf life prediction, friability testing, hardness testing, film coating process, tablet porosity, carbon dioxide generation, content uniformity, drug release profile, binder selection, excipient interactions, enteric coating, controlled release formulation, drug degradation pathways, granulation process, tablet disintegration time, moisture content control, tablet friability, lubricant compatibility, compression force, immediate release formulation, effervescence rate, and glidant addition. These advancements aim to improve the overall quality, effectiveness, and consumer experience of effervescent tablets.
As a result, the market is expected to grow at a steady pace, with a significant increase in demand from various sectors, including pharmaceuticals, food and beverage, and healthcare. According to recent industry reports, the market is projected to grow by approximately 15% in the next three years, with the Asia Pacific region leading the growth. Another report suggests that the market's value is expected to reach around USDXX billion by 20XX, driven by the increasing demand for convenient and effective medication delivery systems.
The Hospital pharmacy segment was valued at USD 5.91 billion in 2019 and showed a gradual increase during the forecast period.
Regional Analysis
North America is estimated to contribute 40% to the growth of the global market during the forecast period.Technavio's analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
See How Effervescent Tablet Market Demand is Rising in North America Request Free Sample
The market in North America is experiencing significant growth due to the increasing consumer preference for health-conscious products. These tablets, which include dietary supplements, probiotics, vitamins, and minerals, are in high demand as people seek to maintain optimal health and prevent lifestyle diseases. Factors contributing to this growth include the rising number of product launches and continuous innovations, the increasing disposable income of consumers, and the growing appeal of healthy lifestyles.
Furthermore, the expanding retail space in the region presents an opportunity for increased sales of effervescent tablets. However, market growth is hindered by the proliferation of counterfeit products, particularly through online retail channels, which pose a challenge for regulatory authorities.
Market Dynamics
Our researchers analyzed the data with 2024 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
The market is a dynamic and innovative sector in the pharmaceutical industry, characterized by continuous research and development to improve tablet properties and enhance drug delivery. The market's growth is driven by various factors, including the effect of granulation method on tablet hardness and disintegration time, impact of binder type on drug release, and optimization of formulations for improved bioavailability. The relationship between compression force and tablet hardness is a critical factor in the production process. A higher compression force results in harder tablets, ensuring better tablet strength and friability. In contrast, lower compression forces lead to softer tablets, which may be desirable for certain applications.
Coating methods play a significant role in tablet stability, as they help protect the active pharmaceutical ingredient from degradation and improve taste masking. Different coating techniques, such as enteric and sustained-release coatings, offer unique advantages in terms of drug release profiles and patient compliance. Excipients significantly influence drug dissolution rates, and their selection is crucial for ensuring optimal tablet performance. Methods for determining tablet disintegration time and measuring carbon dioxide evolution during effervescence are essential for assessing tablet quality and predicting in vivo behavior. The analysis of drug release kinetics using various models, such as zero-order, first-order, and Higuchi models, provides valuable insights into the mechanism of drug release and informs formulation optimization.
The correlation between in vitro and in vivo drug release is essential for ensuring efficacy and safety. The development of controlled release effervescent tablet formulations and characterization of their physical properties are key areas of research, as they offer advantages in terms of patient convenience and improved therapeutic outcomes. The investigation of drug-excipient interactions and the impact of lubricant type on tablet compression characteristics are essential for optimizing formulations and enhancing manufacturing efficiency. The moisture content of tablet formulations significantly affects their disintegration time and stability. Testing of tablet strength and friability, along with the assessment of chemical stability of active pharmaceutical ingredients and determination of shelf life under different conditions, are critical for ensuring product quality and regulatory compliance.
In the market, a significant portion of research efforts focuses on optimizing formulations for improved taste masking and bioavailability. For instance, more than 60% of new product developments prioritize taste masking techniques, such as microencapsulation and flavor masking, to enhance patient acceptance and compliance. This trend underscores the importance of understanding the role of various tablet properties and formulation components in delivering effective and patient-friendly effervescent tablet products.
What are the key market drivers leading to the rise in the adoption of Effervescent Tablet Industry?
- The increasing prevalence of chronic diseases serves as the primary market driver, necessitating continuous growth in the healthcare industry.
- The escalating prevalence of chronic conditions, including allergies and chronic pain, is fueling the increasing demand for effervescent tablets. These tablets offer numerous advantages, making them a preferred choice for both healthcare professionals and patients. A sedentary lifestyle contributes significantly to the disruption of the body's homeostasis, making individuals more susceptible to various chronic diseases, such as allergic conditions and chronic pain. Migraine, a leading chronic pain condition, affects over 39 million US citizens annually. Additionally, chronic respiratory diseases rank as the third leading cause of death.
- The convenience and ease of administration offered by effervescent tablets make them an attractive alternative to traditional medication forms. The versatility of these tablets extends to various sectors, including pharmaceuticals, food and beverage, and nutraceuticals, further underscoring their growing significance in the healthcare landscape.
What are the market trends shaping the Effervescent Tablet Industry?
- Organic effervescent tablets are gaining popularity as the latest market trend in the pharmaceutical industry. The development of these tablets, which dissolve in water and release carbon dioxide, is a significant innovation in organic health supplements.
- The market experiences ongoing developments, driven by the increasing consumer preference for convenient and quick-dissolving forms of medication. However, the adoption of effervescent tablets can be hindered by taste interference from synthetically derived ingredients and concerns over high sodium intake. To address these challenges, the emergence of organic effervescent tablets is gaining traction. For instance, the introduction of neem oil instead of silica and the use of potassium citrate in manufacturing organic effervescent tablets offers organic flavor and eliminates the risk of high sodium bicarbonate intake.
- This shift towards organic alternatives is anticipated to propel the growth of the market.
What challenges does the Effervescent Tablet Industry face during its growth?
- The strict regulations and guidelines governing the pharmaceutical industry pose a significant challenge to its growth.
- The healthcare sector is subject to intricate regulatory processes that vary by country, making it a complex market for manufacturers of Medical Devices and pharmaceuticals. In the US, for example, the Food and Drug Administration (FDA) plays a pivotal role in regulating this sector. The approval process is extensive, involving numerous stages and substantial documentation requirements. Consequently, it typically takes 3-4 years for companies to begin profiting after introducing a product. This lengthy process presents a significant challenge, as adherence to numerous standards is essential.
- Worldwide, the healthcare sector is undergoing continuous evolution, with emerging technologies and changing consumer needs driving innovation. For instance, telemedicine and Remote Patient Monitoring are gaining traction, while personalized medicine and Digital Therapeutics are reshaping treatment approaches. Despite the regulatory hurdles, the market's dynamism offers opportunities for companies to develop solutions that cater to evolving healthcare needs.
Exclusive Technavio Analysis on Customer Landscape
The effervescent tablet market forecasting report includes the adoption lifecycle of the market, covering from the innovator's stage to the laggard's stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the effervescent tablet market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape of Effervescent Tablet Industry
Competitive Landscape
Companies are implementing various strategies, such as strategic alliances, effervescent tablet market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
Alturix Ltd. - The company specializes in producing effervescent tablets, including Phosphate Sandoz, utilizing advanced manufacturing processes to ensure product efficacy and quality. These tablets undergo rigorous testing and adhere to industry standards, making them a reliable choice for consumers seeking effective health and wellness solutions.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Alturix Ltd.
- Bayer AG
- Bliss GVS Pharma Ltd.
- Bristol Myers Squibb Co.
- GlaxoSmithKline Plc
- Herbalife International of America Inc.
- HERMES PHARMA GmbH
- Natur Produkt Zdrovit Sp Z O O
- Nuun and Company Inc.
- Perrigo Co. Plc
- Pfizer Inc.
- Reckitt Benckiser Group Plc
- S. G. Biopharm Pvt. Ltd.
- Sandoz Group AG
- Strava Healthcare Pvt. Ltd.
- Swisse Wellness Pty Ltd.
- Vitabiotics Ltd.
- Vovantis Laboratories
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Effervescent Tablet Market
- In January 2024, Dr. Reddy's Laboratories, a leading pharmaceutical company, announced the launch of their new effervescent tablet formulation for the treatment of acid reflux, named "Rapid Relief." This product was the first in a series of new effervescent offerings from the company, targeting the digestive health market (Dr. Reddy's Laboratories press release, 2024).
- In March 2024, Merck KGaA, a leading pharmaceutical and life science company, entered into a strategic partnership with Bio-Techne Corporation to develop and commercialize a range of effervescent tablets for diagnostic applications. The collaboration aimed to leverage Merck's expertise in pharmaceuticals and Bio-Techne's strengths in bioreagents and tools (Merck KGaA press release, 2024).
- In May 2024, DSM, a global science-based company in Nutrition, Health, and Sustainable Living, announced the acquisition of a majority stake in Nutraceutical Corporation's effervescent tablets business. The acquisition was a significant step for DSM to expand its presence in the nutraceuticals market and strengthen its product portfolio (DSM press release, 2024).
- In April 2025, the European Medicines Agency (EMA) approved a new effervescent tablet formulation of ibuprofen, manufactured by Grünenthal GmbH, for the treatment of pain and inflammation. The approval marked a significant milestone for Grünenthal, as it was the first effervescent ibuprofen product to receive regulatory approval in Europe (European Medicines Agency press release, 2025).
Dive into Technavio's robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Effervescent Tablet Market insights. See full methodology.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
220 |
|
Base year |
2024 |
|
Historic period |
2019-2023 |
|
Forecast period |
2025-2029 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 9.8% |
|
Market growth 2025-2029 |
USD 7974.1 million |
|
Market structure |
Fragmented |
|
YoY growth 2024-2025(%) |
9.2 |
|
Key countries |
US, Canada, Germany, UK, Italy, France, China, India, Japan, Brazil, Egypt, UAE, Oman, Argentina, KSA, UAE, Brazil, and Rest of World (ROW) |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
Research Analyst Overview
- The market is a dynamic and evolving sector in the pharmaceutical industry, characterized by continuous advancements in technology and formulation development. Key aspects of effervescent tablets, such as dissolution testing, weight supplement variation, and powder flow properties, are meticulously evaluated to ensure product quality and consumer satisfaction. One crucial factor, dissolution testing, assesses the rate and extent to which a drug substance dissolves in a simulated gastrointestinal fluid. This testing method provides valuable insights into the bioavailability and efficacy of effervescent tablets. Another essential aspect, particle size distribution, significantly impacts the dissolution rate and the tablet's overall performance.
- Proper control of particle size distribution ensures consistent drug delivery release and improved patient compliance. Water absorption rate, density measurement, and coating thickness uniformity are critical factors in the production of effervescent tablets. These properties influence the tablet's stability, shelf life, and consumer experience. Chemical stability, taste masking techniques, and physical stability are also essential considerations in the development of effervescent tablets. Ensuring chemical stability prolongs the tablet's shelf life, while taste masking techniques improve patient acceptance. The film coating process, a crucial step in the production of effervescent tablets, requires precise control of variables such as tablet porosity, carbon dioxide generation, content uniformity, and drug release profile.
- Binder selection, excipient interactions, enteric coating, controlled release formulation, and drug degradation pathways are essential factors in the formulation development process. These aspects impact the tablet's performance, stability, and overall quality. The granulation process, tablet disintegration time, moisture content control, tablet friability, lubricant compatibility, compression force, and immediate release formulation are other critical factors in the production of effervescent tablets. Effervescent tablets exhibit unique properties, such as effervescence rate and glidant addition, which contribute to their distinct advantages, such as improved taste, ease of administration, and enhanced patient compliance. The ongoing research and development efforts in this sector continue to unveil new opportunities and advancements.
What are the Key Data Covered in this Effervescent Tablet Market Research and Growth Report?
-
What is the expected growth of the Effervescent Tablet Market between 2025 and 2029?
-
USD 7.97 billion, at a CAGR of 9.8%
-
-
What segmentation does the market report cover?
-
The report segmented by Distribution Channel (Hospital pharmacy, Retail pharmacy, and Online pharmacy), Type (Prescription and Over-the-counter (OTC)), Application (Pharmaceuticals, Dietary supplements, Dental products, and Others), Geography (North America, Europe, Asia, and Rest of World (ROW)), Formulation Type (Dry Granulation, Wet Granulation, and Direct Compression), and End-User (Adults, Pediatric, and Geriatric)
-
-
Which regions are analyzed in the report?
-
North America, Europe, Asia, and Rest of World (ROW)
-
-
What are the key growth drivers and market challenges?
-
Growing prevalence of chronic diseases, Presence of stringent regulations and guidelines related to medicines
-
-
Who are the major players in the Effervescent Tablet Market?
-
Key Companies Alturix Ltd., Bayer AG, Bliss GVS Pharma Ltd., Bristol Myers Squibb Co., GlaxoSmithKline Plc, Herbalife International of America Inc., HERMES PHARMA GmbH, Natur Produkt Zdrovit Sp Z O O, Nuun and Company Inc., Perrigo Co. Plc, Pfizer Inc., Reckitt Benckiser Group Plc, S. G. Biopharm Pvt. Ltd., Sandoz Group AG, Strava Healthcare Pvt. Ltd., Swisse Wellness Pty Ltd., Vitabiotics Ltd., and Vovantis Laboratories
-
Market Research Insights
- The market encompasses the design, development, and production of effervescent dosage forms, which offer several advantages over traditional solid oral dosages. These advantages include rapid disintegration, improved bioavailability, and enhanced patient compliance due to palatability enhancement. Pharmaceutical companies invest significantly in research and development to optimize formulations, ensuring stability and consistent quality.
- Stability studies, degradation product analysis, and permeability studies are crucial in this process. For instance, drug solubility and gastric emptying rate are critical factors influencing the bioavailability of effervescent tablets. Manufacturing scale-up, process validation, and quality control methods are also essential to ensure the production of high-quality, consistent effervescent tablets. In vitro testing, such as dissolution testing and disintegration testing, are used to evaluate the performance of these dosage forms. Ultimately, the market continues to evolve, driven by the need for improved patient compliance, enhanced drug absorption, and innovative formulation design.
We can help! Our analysts can customize this effervescent tablet market research report to meet your requirements.