Implantable Loop Recorders Market Size 2024-2028
The implantable loop recorders market size is forecast to increase by USD 532.7 million at a CAGR of 6.78% between 2023 and 2028.
- The Implantable Loop Recorder (ILR) market is witnessing significant growth due to the increasing prevalence of cardiac conditions, particularly those related to irregular heart rhythms and chronic diseases. According to the American Heart Association, an estimated 12 million Americans are living with atrial fibrillation, a common arrhythmia that can lead to the need for an ILR. Furthermore, supportive government and non-government initiatives are driving market expansion. For instance, the European Union's Horizon 2020 research and innovation program has funded several projects aimed at developing advanced ILRs. However, the ILR market faces challenges due to stringent regulations.
- These regulations require extensive clinical trials and rigorous testing to ensure the safety and efficacy of these devices. Additionally, the high cost of ILRs and the need for frequent follow-ups and battery replacements can limit their widespread adoption. Companies seeking to capitalize on market opportunities must navigate these challenges effectively by investing in research and development to meet regulatory requirements and reduce costs. Furthermore, partnerships with healthcare providers and insurance companies can help make ILRs more accessible and affordable for patients.
What will be the Size of the Implantable Loop Recorders Market during the forecast period?
- The implantable loop recorder market continues to evolve, driven by advancements in technology and shifting healthcare trends. These devices, used for arrhythmia monitoring and electrophysiological studies, are increasingly being adopted in outpatient settings and precision medicine, enabling personalized treatment plans and remote monitoring. Healthcare IT plays a crucial role in integrating patient data from these devices with electronic health records, facilitating value-based healthcare and data-driven clinical decision-making. Regulatory compliance and data security are paramount in this sector, with regulatory bodies and healthcare systems demanding stringent data privacy and interoperability standards. Machine learning and artificial intelligence are being leveraged to enhance diagnostic accuracy and predictive analytics, while wearable technology and mobile health solutions offer new opportunities for patient engagement and home monitoring.
- Implantable loop recorders are also being integrated with therapeutic devices, such as cardiac ablation systems, to provide a more comprehensive solution for managing heart failure and cardiac arrhythmias. Research and development efforts are ongoing, with clinical trials exploring new applications and data analysis techniques. The healthcare market's continuous dynamism underscores the importance of patient compliance, data acquisition, and data analysis in delivering effective, patient-centric care.
How is this Implantable Loop Recorders Industry segmented?
The implantable loop recorders industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2024-2028, as well as historical data from 2018-2022 for the following segments.
- Application
- Cardiac arrhythmia
- Atrial fibrillation
- Type
- Automatic
- Manual
- End-User
- Hospitals
- Cardiac Centers
- Hospitals
- Cardiac Centers
- Geography
- North America
- US
- Canada
- Europe
- Denmark
- Spain
- APAC
- China
- Rest of World (ROW)
- North America
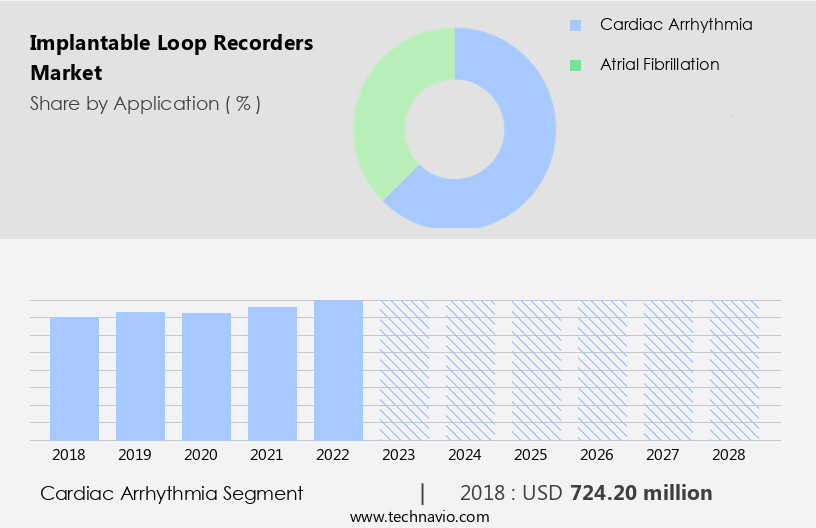
By Application Insights
The cardiac arrhythmia segment is estimated to witness significant growth during the forecast period.
In the realm of healthcare, the implantable loop recorder emerges as a vital diagnostic tool in managing cardiac arrhythmia, an irregular heartbeat caused by dysfunctional electric signals coordinating heartbeats. This condition can manifest as either tachycardia or bradycardia. The implantable loop recorder, a small, battery-operated device, enables healthcare professionals to remotely monitor a patient's heart rhythm for extended durations, typically up to three years. This extended monitoring period is crucial for accurately diagnosing the root cause of cardiac arrhythmia, which may not always be evident during routine electrocardiograms. The outpatient setting is a common location for implanting these devices, ensuring patient-centric care and reducing hospitalization costs.
Incorporating healthcare IT and data integration, patient data is seamlessly analyzed, enabling personalized treatment plans and value-based healthcare. Remote monitoring facilitates real-time data acquisition and analysis, enhancing diagnostic accuracy and patient engagement. Arrhythmia monitoring plays a significant role in managing cardiac arrhythmia, with atrial fibrillation and ventricular tachycardia being common conditions. Clinical trials and patient data management are essential for advancing research and development in this field. Loop recording technology, a critical component of implantable loop recorders, is integrated with machine learning and artificial intelligence to improve diagnostic accuracy and device interoperability. Regulatory compliance and data security are paramount in this digital health landscape.
Wearable technology and home monitoring systems offer additional avenues for remote healthcare, while predictive analytics and data analysis provide insights into patient trends and potential health risks. Healthcare data, a valuable asset, is integrated into electronic health records for comprehensive patient care. Cardiac ablation and therapeutic devices are essential components of the healthcare market, working in tandem with implantable loop recorders to improve patient outcomes. Research and development in this sector continue to evolve, with a focus on improving diagnostic accuracy, device interoperability, and patient engagement.
The Cardiac arrhythmia segment was valued at USD 724.20 million in 2018 and showed a gradual increase during the forecast period.
Regional Analysis
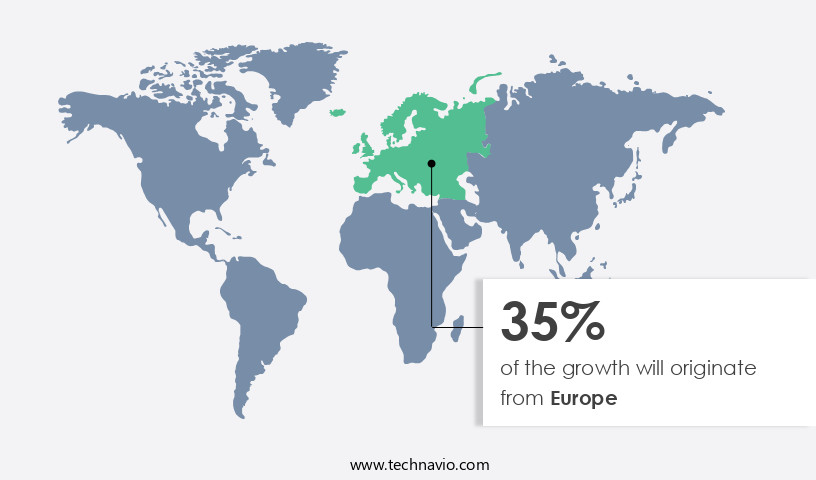
Europe is estimated to contribute 35% to the growth of the global market during the forecast period.Technavio’s analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
The implantable loop recorder market is experiencing significant growth in the North American region due to the advanced healthcare infrastructure, increasing awareness about cardiac monitoring devices, and a high prevalence of cardiovascular diseases (CVDs). The US and Canada are the major contributors to the market in North America, driven by high healthcare expenditure on CVDs, a growing geriatric population, and the adoption of technologically advanced implantable loop recorders. The market's expansion is further fueled by the rise in remote monitoring, value-based healthcare, and patient-centric care. In the outpatient setting, these devices enable healthcare professionals to monitor cardiac arrhythmias, such as atrial fibrillation and ventricular tachycardia, in real-time.
Machine learning and artificial intelligence technologies are integrated into these devices for predictive analytics, improving diagnostic accuracy and patient engagement. Data privacy and security are prioritized with robust data integration and data analysis capabilities. The market's growth is also supported by regulatory compliance, clinical trials, and research & development in therapeutic devices and cardiac ablation. Digital health, healthcare IT, and mobile health technologies facilitate data acquisition, data analysis, and patient education, enhancing the overall effectiveness of implantable loop recorders. Wireless technology and wearable technology are also transforming the market, enabling home monitoring and remote healthcare.
The implantable loop recorder market is poised for continued growth, driven by the integration of healthcare data, electrophysiological studies, and personalized medicine, with a focus on diagnostic devices, medical devices, and healthcare data.
Market Dynamics
Our researchers analyzed the data with 2023 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
What are the key market drivers leading to the rise in the adoption of Implantable Loop Recorders Industry?
- The increasing prevalence of cardiac conditions, particularly those caused by irregular heart rhythms and chronic diseases, serves as the primary driver for the market's growth.
- The implantable loop recorder market is experiencing significant growth due to the increasing prevalence of cardiac arrhythmias and structural heart diseases. Arrhythmias, such as ventricular fibrillation, can lead to cardiac arrest by causing the heart's lower chambers to stop beating effectively. According to the Centers for Disease Control and Prevention (CDC), approximately 209,000 people in the US receive treatment for in-hospital cardiac arrest each year. Structural heart diseases, including hypertrophic cardiomyopathy, a genetically inherited condition affecting 1 in every 500 people in the UK, can also contribute to arrhythmias. Implantable loop recorders play a crucial role in the diagnosis and treatment of these conditions by continuously monitoring the heart's electrical activity.
- Precision medicine and personalized treatment plans are becoming increasingly important in healthcare, and remote monitoring through implantable loop recorders allows for real-time data collection and analysis. This data can be transmitted to healthcare IT systems for regulatory compliance and value-based healthcare initiatives. Moreover, mobile health technologies enable remote monitoring and real-time data analysis, enhancing the patient experience and improving clinical outcomes. Arrhythmia monitoring through implantable loop recorders is a critical component of electrophysiological studies, ensuring accurate diagnosis and effective treatment. The market's growth is further driven by the emphasis on regulatory compliance and the integration of these technologies into the healthcare ecosystem.
What are the market trends shaping the Implantable Loop Recorders Industry?
- The emerging market trend reflects a strong emphasis on both government and non-government initiatives in providing support. These initiatives are crucial for various sectors and are expected to significantly influence market growth.
- In the global healthcare market, there is a growing emphasis on improving patient care and diagnosis, particularly in the area of cardiovascular diseases such as atrial fibrillation and ventricular tachycardia. Implantable loop recorders (ILRs) have emerged as a crucial diagnostic device in this context, offering continuous monitoring of cardiac activity. Patient compliance is a significant challenge in long-term monitoring, and ILRs address this issue by providing reliable and accurate data. Data integration from these devices is essential for effective patient data management and clinical trials. The healthcare system's digital transformation has opened up opportunities for machine learning algorithms to analyze the vast amounts of data generated by ILRs, leading to improved diagnostic accuracy and personalized treatment plans.
- Governments and healthcare organizations worldwide are investing in the development of their healthcare infrastructure and increasing access to quality care. The focus is on making healthcare affordable and accessible to all, especially in underpenetrated areas. In the context of cardiovascular diseases, collaboration between the healthcare industry and organizations is vital in addressing the rising number of cases and improving patient outcomes. In conclusion, the implementation of advanced diagnostic devices like ILRs and data integration technologies is crucial in enhancing patient care and improving healthcare systems' efficiency. The ongoing digital health revolution and the potential of machine learning algorithms to analyze vast amounts of data offer significant opportunities for innovation and improvement in the cardiovascular disease diagnosis and management landscape.
What challenges does the Implantable Loop Recorders Industry face during its growth?
- The stringent regulations governing the implantation and use of loop recorders pose a significant challenge to the growth of the industry. These regulations, which aim to ensure patient safety and device effectiveness, necessitate rigorous testing, approval processes, and ongoing compliance. Adhering to these requirements adds complexity and cost to the development and market introduction of loop recorders, potentially hindering the industry's expansion.
- Implantable medical devices, such as those used for heart failure management, play a significant role in patient-centric care by enabling continuous data acquisition and remote healthcare monitoring. These devices, including implantable loop recorders, are subjected to stringent regulations to ensure data privacy and patient safety. Classified as class III medical devices by the US Food and Drug Administration (FDA), they undergo a rigorous pre-market approval (PMA) process. This process involves clinical trials, extensive testing, and significant financial investment, with costs estimated to be around USD250,000 in 2015.
- Healthcare professionals rely on these devices to provide valuable data for research and development of therapeutic devices and electronic health records. The integration of these devices with healthcare data systems allows for more efficient and effective patient care, ultimately improving overall healthcare outcomes.
Exclusive Customer Landscape
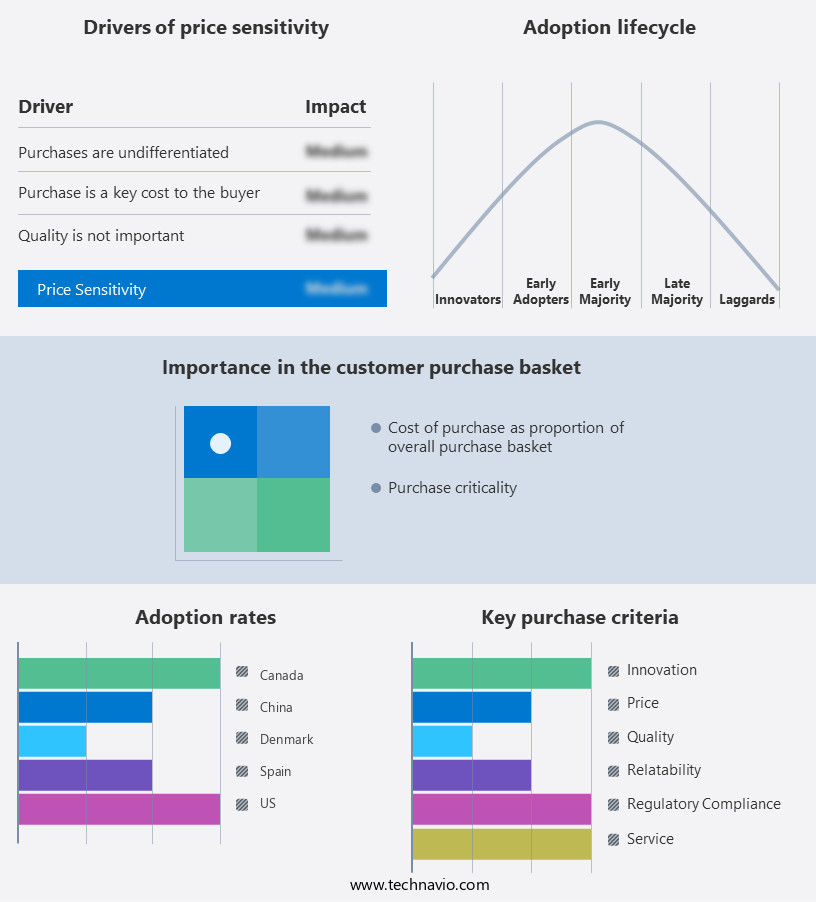
The implantable loop recorders market forecasting report includes the adoption lifecycle of the market, covering from the innovator’s stage to the laggard’s stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the implantable loop recorders market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape
Key Companies & Market Insights
Companies are implementing various strategies, such as strategic alliances, implantable loop recorders market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
Abbott Laboratories - The company introduces the Jot Dx, an advanced implantable loop recorder for continuous cardiac monitoring. This innovative medical device provides accurate and reliable data for diagnosing and managing heart rhythm disorders. The Jot Dx is a small, battery-powered device that is implanted under the skin, enabling long-term, uninterrupted monitoring. Its compact design ensures patient comfort and minimal invasiveness. The device records and transmits cardiac data to healthcare providers for analysis, facilitating timely and effective treatment plans. The Jot Dx's advanced technology ensures high-quality data, enabling early detection and intervention for various heart conditions. This cutting-edge solution empowers healthcare professionals to deliver personalized care, improving patient outcomes and quality of life.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Abbott Laboratories
- Angel Medical Systems Inc.
- Asahi Kasei Corp.
- BIOTRONIK SE and Co. KG
- Bittium Corp.
- Boston Scientific Corp.
- Koninklijke Philips N.V.
- Medtronic Plc
- Vectorious Medical Technologies Ltd.
- SunTech Medical Inc.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Implantable Loop Recorders Market
- In February 2024, Medtronic, a leading medical technology company, announced the FDA approval of their new Reveal LINQ II Insertable Cardiac Monitor (ICM). This device is smaller and thinner than its predecessor, enabling easier insertion and improved patient comfort (Medtronic Press Release, 2024).
- In July 2025, Boston Scientific and Philips entered into a strategic partnership to integrate Philips' remote monitoring and analytics solutions with Boston Scientific's implantable loop recorders. This collaboration aims to enhance patient care and improve diagnostic accuracy (Boston Scientific Press Release, 2025).
- In October 2024, Abbott Laboratories completed the acquisition of St. Jude Medical, a major player in the market. This merger expanded Abbott's cardiac rhythm management portfolio and strengthened its position in the global market (Abbott Laboratories SEC Filing, 2024).
- In March 2025, the European Commission approved the use of the Medtronic Reveal LINQ II ICM in the European Union. This approval marked the device's entry into the European market, expanding its reach and potential customer base (Medtronic Press Release, 2025).
Research Analyst Overview
The implantable loop recorder market within cardiac rhythm management is experiencing significant growth due to the increasing prevalence of chronic heart disease. These devices, which include implantable cardiac monitors, offer long-term monitoring for patients at risk of cardiovascular disease. Device durability and complications management are crucial factors in ensuring patient safety and quality of life. Remote patient management and automated arrhythmia detection enable healthcare providers to closely monitor patients and provide early warning systems for interventional cardiology. Medical data analytics and patient follow-up facilitate clinical effectiveness and cost savings.
Device safety, calibration, battery life, and programming are essential for optimal device performance. Healthcare outcomes are improved through loop recorder technology, which provides valuable information for electrophysiology monitoring. The economic impact of these devices is significant, as they reduce healthcare costs by enabling early intervention and improving patient care.
Dive into Technavio’s robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Implantable Loop Recorders Market insights. See full methodology.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
156 |
|
Base year |
2023 |
|
Historic period |
2018-2022 |
|
Forecast period |
2024-2028 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 6.78% |
|
Market growth 2024-2028 |
USD 532.7 million |
|
Market structure |
Concentrated |
|
YoY growth 2023-2024(%) |
6.23 |
|
Key countries |
US, Canada, Spain, Denmark, and China |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
What are the Key Data Covered in this Implantable Loop Recorders Market Research and Growth Report?
- CAGR of the Implantable Loop Recorders industry during the forecast period
- Detailed information on factors that will drive the growth and forecasting between 2024 and 2028
- Precise estimation of the size of the market and its contribution of the industry in focus to the parent market
- Accurate predictions about upcoming growth and trends and changes in consumer behaviour
- Growth of the market across North America, Europe, Asia, and Rest of World (ROW)
- Thorough analysis of the market’s competitive landscape and detailed information about companies
- Comprehensive analysis of factors that will challenge the implantable loop recorders market growth of industry companies
We can help! Our analysts can customize this implantable loop recorders market research report to meet your requirements.