Mri Compatible IV Infusion Pumps Market Size 2024-2028
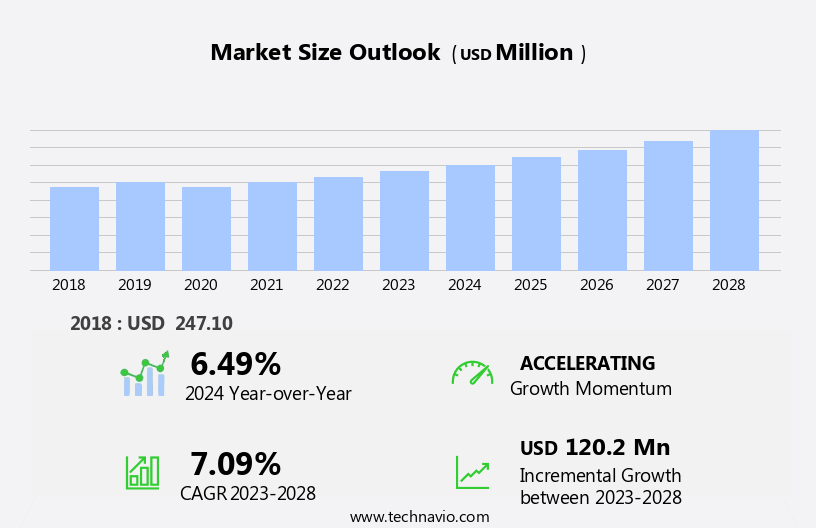
The MRI compatible IV infusion pumps market size is forecast to increase by USD 120.2 million at a CAGR of 7.09% between 2023 and 2028.
- The market witnesses significant growth driven by the increasing prevalence of chronic diseases, particularly cancer and neurological disorders, which necessitate frequent MRI scans and the use of specialized infusion pumps during the imaging process. This trend is further amplified in emerging countries, where the burden of chronic diseases is on the rise and healthcare infrastructure is expanding. However, the market faces challenges, including high costs associated with these advanced pumps, which can limit their adoption in resource-constrained settings. Additionally, regulatory hurdles impact adoption, as these pumps must meet stringent safety and performance standards to ensure patient safety during MRI scans.
- To capitalize on market opportunities and navigate challenges effectively, companies must focus on developing cost-effective solutions while ensuring regulatory compliance and addressing supply chain inconsistencies to ensure a steady flow of high-quality products to meet growing demand.
What will be the Size of the MRI compatible IV infusion Pumps Market during the forecast period?
- The market is experiencing significant growth, driven by the increasing demand for advanced medical devices that can function in magnetic resonance imaging (MRI) environments. These pumps are essential for delivering antibiotic therapy and other medications during MRI scans, addressing the challenge of gradient fields that can interfere with traditional infusion systems. Data analytics and pharmacist integration are key trends in this market, enabling real-time monitoring of fluid management, drug utilization review, compliance monitoring, and pharmacokinetic modeling. Hospital information systems and patient portals are increasingly integrating these smart infusion pumps, allowing for medication reconciliation and personalized therapy protocols. Non-magnetic materials and device integration are crucial considerations for MRI-compatible pumps, ensuring minimal electromagnetic interference and wireless communication.
- Cloud-based platforms and RF pulses enable remote access to patient data and real-time adjustments to drug administration protocols. Image artifact reduction and pain management are additional features that enhance the functionality of these pumps, making them indispensable for hospitals and healthcare providers. Blood transfusion and IV therapy applications further expand their utility, ensuring optimal electrolyte balance and patient safety.
How is this MRI Compatible IV Infusion Pumps Industry segmented?
The MRI compatible IV infusion pumps industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2024-2028, as well as historical data from 2018-2022 for the following segments.
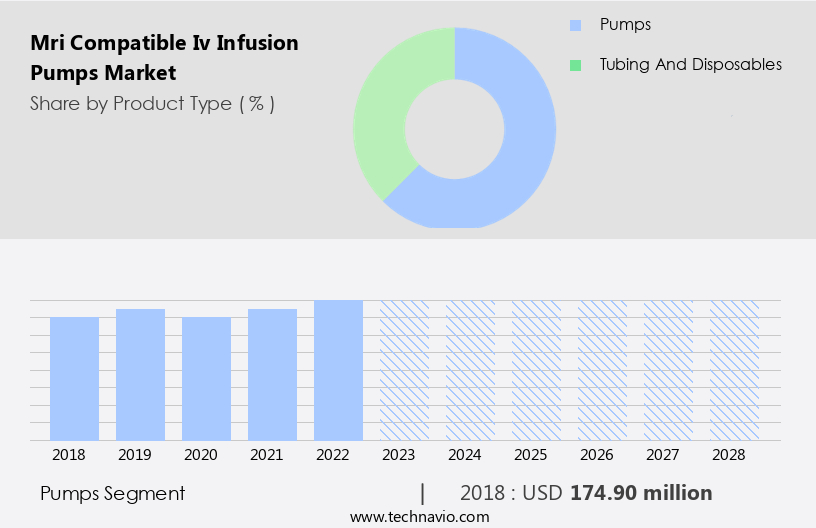
- Product Type
- Pumps
- Tubing and disposables
- Geography
- North America
- US
- Canada
- Europe
- France
- Germany
- APAC
- China
- Rest of World (ROW)
- North America
By Product Type Insights
The pumps segment is estimated to witness significant growth during the forecast period.
In the healthcare sector, large-volume infusion pumps are a crucial component in delivering medications and nutrients to patients in various settings, including hospitals and clinical facilities. These pumps, which primarily use linear peristaltic technology, are integral to administering large volumes of fluids, such as saline solutions, antibiotics, and nutrients. The expanding prevalence of chronic diseases and the increasing demand for outpatient care contribute significantly to the rising adoption of large-volume infusion pumps. Safety standards are paramount in the medical devices industry, and infusion pumps are no exception. Compliance with regulatory bodies is essential, particularly in hospital settings where patient safety is a top priority.
MRI compatibility is a critical consideration for infusion pumps used in clinical settings, enabling seamless integration with medical imaging equipment for image-guided therapy. Patient comfort is another essential factor in the design and functionality of infusion pumps. Patient mobility and wireless connectivity have become increasingly important, allowing for ambulatory infusion and remote monitoring. Precision medicine and automated dispensing systems further enhance the accuracy and efficiency of drug delivery, reducing errors and improving patient outcomes. In critical care settings, infusion pumps play a vital role in intensive care units, where multi-drug administration, bolus administration, and flow rate control are essential.
Syringe pumps are also widely used for precise drug delivery, especially in emergency medicine. Data management and alarm systems are integral to ensuring quality of care and patient safety, providing real-time monitoring and alerts for potential issues. The integration of electronic health records and remote monitoring capabilities further enhances the functionality and utility of infusion pumps in clinical trials and patient care. Infusion pumps continue to evolve, incorporating advanced features such as volume control, dosage calculation, and drug library management, to meet the evolving needs of healthcare providers and patients.
The Pumps segment was valued at USD 174.90 million in 2018 and showed a gradual increase during the forecast period.
Regional Analysis
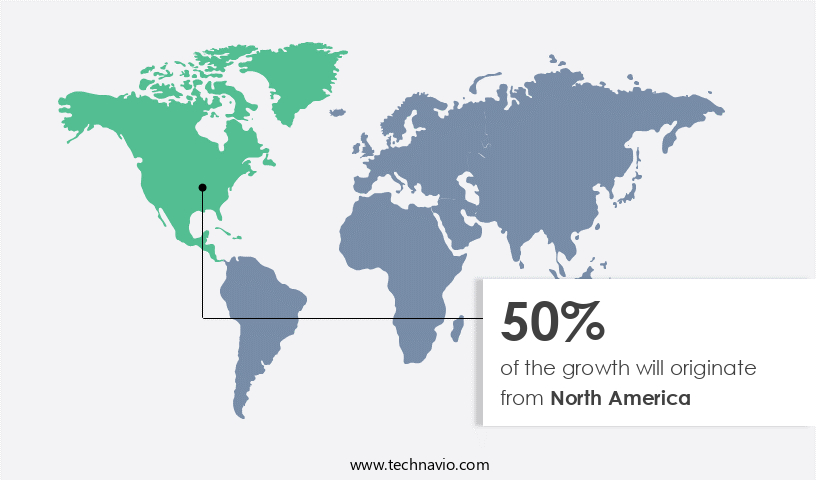
North America is estimated to contribute 50% to the growth of the global market during the forecast period. Technavio's analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
The market in North America experienced significant growth in 2023, with the US and Canada being the major contributors. This expansion is attributed to the increasing geriatric population, the strong presence of established companies, and the rising prevalence of diseases such as cancer, chronic conditions, inflammatory bowel disease (IBD), end-stage renal disease (ESRD), and urinary incontinence. Nearly one in ten Canadians experience urinary incontinence. In response, the market is witnessing increased adoption of MRI compatible IV infusion pumps to ensure patient comfort and safety during magnetic resonance imaging procedures. These pumps enable uninterrupted drug delivery and are essential in clinical settings, emergency medicine, intensive care, and critical care.
The market is further propelled by regulatory compliance, precision medicine, and the integration of advanced features such as automated dispensing, volume control, dosage calculation, bolus administration, and syringe pumps. Additionally, the integration of wireless connectivity, error reduction, patient safety, data management, and image-guided therapy enhances the quality of care and clinical trials. Medical devices manufacturers are focusing on developing multi-drug administration systems, diagnostic imaging, and flow rate control to cater to the diverse needs of patients and healthcare providers.
Market Dynamics
Our researchers analyzed the data with 2023 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
What are the MRI Compatible IV Infusion Pumps market drivers leading to the rise in the adoption of Industry?
- The escalating incidence of chronic diseases serves as the primary catalyst for market growth.
- Infusion pumps play a crucial role in delivering precise and continuous medication to patients, particularly those with chronic conditions such as diabetes, arthritis, cancer, and obesity. The increasing prevalence of these diseases necessitates long-term treatment with therapeutic medications. Patient mobility and wireless connectivity are essential features in modern infusion pumps, enabling patients to move around while receiving treatment. Error reduction is another significant advantage of advanced infusion pumps, ensuring patient safety and minimizing the risk of medication errors. Data management is a critical aspect of infusion pump technology, allowing healthcare providers to monitor and analyze patient data in real-time.
- Syringe pumps and drug library systems facilitate accurate dosage calculation and administration, ensuring optimal treatment outcomes. Intensive care units (ICUs) heavily rely on infusion pumps to manage critically ill patients, making their functionality and reliability indispensable. Infusion pumps are evolving to meet the demands of modern healthcare, integrating advanced features such as wireless connectivity, error reduction, and data management systems. These advancements contribute to improved patient safety, enhanced patient care, and more efficient healthcare delivery.
What are the Mri Compatible Iv Infusion Pumps market trends shaping the Industry?
- Emerging countries present a significant growth potential that is currently a prominent market trend. Businesses seeking expansion opportunities should consider investing in these regions.
- In the realm of medical devices, Mri Compatible Iv Infusion Pumps hold considerable importance, particularly in the context of bolus administration and multi-drug infusion in critical care settings. These devices are essential for maintaining precise flow rate control during diagnostic imaging, image-guided therapy, and ambulatory infusion. The global market for Mri Compatible Iv Infusion Pumps is experiencing significant growth, driven by the increasing number of clinical trials and the need for improved quality of care. Developing and emerging economies, such as Brazil, Russia, India, China, Saudi Arabia, and Mexico, represent substantial opportunities for market expansion.
- With a population that is aging and a growing middle-class community, these countries are investing heavily in healthcare infrastructure and services. The increasing prevalence of chronic diseases, such as obesity, diabetes, and heart disease, further underscores the need for advanced medical devices like Mri Compatible Iv Infusion Pumps.
How does MRI Compatible IV Infusion Pumps market faces challenges face during its growth?
- The high costs linked to MRI-compatible IV infusion pumps pose a significant challenge to the industry's growth trajectory. These expenses, which are a necessary investment for healthcare facilities to offer magnetic resonance imaging services without interfering with patients' intravenous treatments, can limit the expansion and accessibility of MRI technology.
- MRI compatible IV infusion pumps are essential medical devices used primarily in clinical and hospital settings to deliver medications or nutrients to patients undergoing Magnetic Resonance Imaging (MRI) procedures. These pumps are designed to operate safely in the magnetic environment of an MRI machine, ensuring patient comfort and safety. The integration of advanced features, such as electronic health records compatibility, remote monitoring, and automated dispensing, enhances the functionality and efficiency of these devices. Safety is a significant concern in the use of IV infusion pumps, particularly in clinical settings. MRI compatible IV infusion pumps adhere to stringent safety standards, including alarm systems that alert healthcare professionals to potential issues, such as infusion rate errors or pump malfunctions.
- The user interface of these pumps is controlled through software, enabling healthcare professionals to maintain the correct infusion rate and optimize patient care. In summary, MRI compatible IV infusion pumps offer numerous benefits, including increased safety, improved patient comfort, and enhanced functionality, making them an essential tool for healthcare professionals in various clinical and hospital settings.
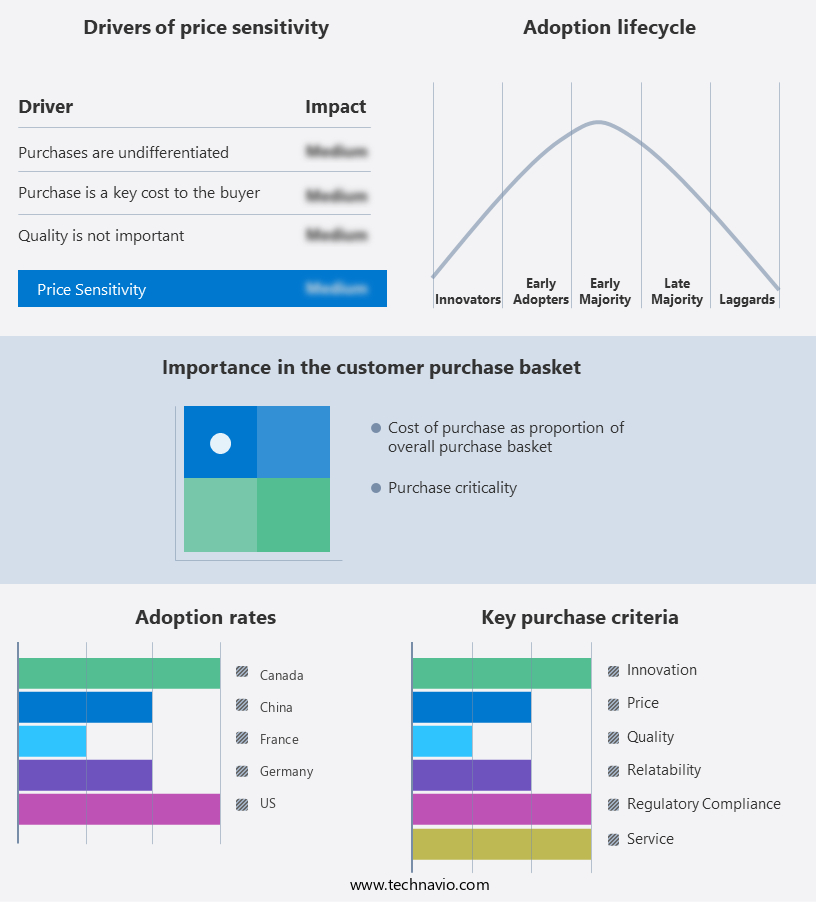
Exclusive Customer Landscape
The MRI-compatible IV infusion pumps market forecasting report includes the adoption lifecycle of the market, covering from the innovator's stage to the laggard's stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the MRI-compatible IV infusion pumps market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape
Key Companies & Market Insights
Companies are implementing various strategies, such as strategic alliances, MRI compatible IV infusion pumps market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
ADOX SA - This company specializes in providing MRI-compatible IV infusion pumps, including the innovative Unique MRI shield model.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- ADOX SA
- Arcomed AG
- B.Braun SE
- Baxter International Inc.
- Becton Dickinson and Co.
- Beijing KellyMed Co. Ltd.
- CODAN ARGUS AG
- Digicare Biomedical Technology Inc.
- Eitan Medical Ltd.
- Flowonix Medical Inc.
- Fresenius SE and Co. KGaA
- ICU Medical Inc.
- IRadimed Corp.
- Medtronic Plc
- Nipro Corp.
- Shenzhen Mindray BioMedical Electronics Co. Ltd
- Terumo Europe NV
- vTitan Corp. Pvt Ltd.
- Zyno Medical
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Mri Compatible Iv Infusion Pumps Market
- In February 2024, Medtronic, a leading medical technology company, announced the FDA approval of its new MRI-conditional Infuseco MP Infusion System. This system, designed for use during MRI scans, eliminates the need for patients to disconnect from their infusion pumps during imaging procedures (Medtronic Press Release, 2024).
- In March 2025, B.Braun, a global medical device manufacturer, entered into a strategic partnership with GE Healthcare to develop MRI-compatible infusion pumps. This collaboration aims to integrate GE Healthcare's MRI technology into B.Braun's infusion systems, enhancing patient safety and convenience (B.Braun Press Release, 2025).
- In May 2024, Fresenius Kabi, a global healthcare company, received CE Mark approval for its Space Infusor MRI, an MRI-conditional infusion pump. This approval enables the company to expand its product offerings in Europe and cater to the growing demand for MRI-compatible medical devices (Fresenius Kabi Press Release, 2024).
- In August 2025, Hospira, a Pfizer company, unveiled its Plum A+ MRI Infusion System, featuring advanced safety features and MRI compatibility. This system's launch marks Hospira's entry into the MRI-compatible infusion pumps market, broadening its product portfolio and addressing the needs of hospitals and clinics (Pfizer Press Release, 2025).
Research Analyst Overview
The market continues to evolve, driven by the growing demand for advanced medical devices that can be used in magnetic resonance imaging (MRI) environments. These pumps are essential in various sectors, including clinical trials, critical care, and ambulatory infusion, to ensure precise drug delivery and improve patient outcomes. Bolus administration, a critical application of MRI compatible infusion pumps, enables the delivery of large doses of medication in a short period, enhancing clinical effectiveness. Medical devices, such as syringe pumps and electronic infusion pumps, offer flow rate control and volume dispensing capabilities, ensuring accurate dosing and reducing errors. MRI compatibility is a crucial factor in the market's dynamics, as the use of MRI imaging is increasingly common in various clinical settings.
Infusion pumps that can operate in MRI environments without interfering with the imaging process are in high demand. Quality of care is a primary concern in the healthcare industry, and MRI compatible infusion pumps contribute significantly to enhancing patient care. These pumps offer wireless connectivity, enabling remote monitoring and data management, which is particularly useful in intensive care settings. The market's ongoing activities are shaped by regulatory compliance and safety standards. MRI compatible infusion pumps must meet stringent safety and performance requirements to ensure patient safety and minimize risks. Multi-drug administration and image-guided therapy are emerging applications of MRI compatible infusion pumps.
These applications offer significant benefits, including improved patient mobility and enhanced precision in drug delivery, contributing to better patient outcomes. The market is a dynamic and evolving sector, driven by the growing demand for advanced medical devices that can operate in MRI environments. Applications such as bolus administration, medical devices, ambulatory infusion, quality of care, clinical trials, critical care, multi-drug administration, diagnostic imaging, flow rate control, and image-guided therapy continue to shape the market's activities, ensuring the delivery of high-quality patient care while maintaining regulatory compliance and safety standards.
Dive into Technavio's robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled MRI Compatible IV Infusion Pumps Market insights. See full methodology.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
138 |
|
Base year |
2023 |
|
Historic period |
2018-2022 |
|
Forecast period |
2024-2028 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 7.09% |
|
Market growth 2024-2028 |
USD 120.2 million |
|
Market structure |
Fragmented |
|
YoY growth 2023-2024(%) |
6.49 |
|
Key countries |
US, Germany, China, France, and Canada |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
What are the Key Data Covered in this Mri Compatible Iv Infusion Pumps Market Research and Growth Report?
- CAGR of the Mri Compatible Iv Infusion Pumps industry during the forecast period
- Detailed information on factors that will drive the growth and forecasting between 2024 and 2028
- Precise estimation of the size of the market and its contribution of the industry in focus to the parent market
- Accurate predictions about upcoming growth and trends and changes in consumer behaviour
- Growth of the market across North America, Europe, Asia, and Rest of World (ROW)
- Thorough analysis of the market's competitive landscape and detailed information about companies
- Comprehensive analysis of factors that will challenge the mri compatible iv infusion pumps market growth of industry companies
We can help! Our analysts can customize this mri compatible iv infusion pumps market research report to meet your requirements.